
To understand diet and health, we should compare apples not to cakes, cookies, and cream, but to oranges.
The Game Changes: Critics Move the Goalposts
Plant-based diets have no doubt seen an explosion in popularity over the last decade. This has often been attributed in part to the success of several mainstream documentary films. Over the last two years, the documentary The Game Changers has received much attention, and has even been cited for helping to spearhead the more recent rise in interest in plant-based diets. Directed by Louie Psihoyos, produced by James Wilks, and released worldwide on Netflix, the documentary explores many of the documented benefits of plant-based diets on health and athletic performance. It is also backed by many cinema and sports legends, including James Cameron, Arnold Schwarzenegger, Jackie Chan, Sir Lewis Hamilton, Novak Djokovic, and Chris Paul. The documentary even prompted Guinness World Records to publish an article on 11 world record-breaking athletes who adhere to predominantly plant-based diets (1). However, as is common with the success of any form of media promoting plant-based diets, the documentary prompted fierce criticism, particularly from the advocates of popular diets rich in animal foods, including the so-called Keto, Paleo, and Carnivore diets. The main argument of the critics is that the studies cited to support the benefits of a plant-based diet are "cherry-picked" outliers, of inadequate quality to dispute the narrative of the benefits of a diet rich in animal foods for optimal health and physical performance.
The critics of plant-based diets have ignored a very substantial body of high-quality evidence of the benefits of a plant-based diet for optimal health and physical performance, including data from over 100 million person-years of follow-up from clinical, genetic, and epidemiological studies, over 1,000 controlled dietary experiments, and thousands of experiments in over 100 species and breeds of animals, including more than 20 species of primates. As with all documentaries that have examined the health benefits of a plant-based diet, the runtime of The Game Changers is far too short to plausibly expect that all lines of evidence could have been documented in any great detail. This review will therefore examine the evidence from the scientific literature to evaluate some of the most well-documented health benefits of a plant-based diet, with a particular focus on those explored in the documentary. In addition, this review will examine how both the certainty of evidence, and the evidence of the magnitude of adverse effects of diets rich in animal foods compare to other harmful environmental and lifestyle exposures, namely cigarette smoking. Furthermore, this review will examine the serious flaws of a number of key arguments and talking points of the advocates of popular animal-based diets, and how ultimately, they are arguing the necessity of idly waiting for a definitive dietary trial they know will likely never be carried out before their narrative can be refuted.

Literally Comparing Apples to Oranges
Menthol cigarette smoking was not associated with an increased risk of either total mortality or cardiovascular disease incidence, and actually associated with a decreased risk of both lung and total cancer incidence in a meta-analysis of 15 studies (2). This was, however, when compared to nonmenthol cigarette smoking. When compared to never-smokers, epidemiological evidence indicates that both menthol and nonmenthol cigarette smokers have a more than two-fold increased risk of premature death (2). Therefore, as a matter of public interest, recommendations to avoid all forms of tobacco smoking are made in consideration of the evidence for comparisons with the most optimal exposure- that of never-smoking. The critics of plant-based diets, however, have chosen to take the exact opposite approach. They argue against restricting the intake of animal foods by citing the findings of low or no correlation with adverse health outcomes compared to predominantly suboptimal foods.
Humans closely regulate energy intake over time, and will generally maintain caloric balance either consciously or unconsciously by compensating for changes in one source of energy with changes in other sources of energy (3). Thus, as a higher intake of one food implies a lower intake of other foods in order to maintain caloric balance, the effect that any specific food, macronutrient, or dietary pattern has on health outcomes can normally only be determined by what sources of energy are being displaced (3 4). As the intake of unrefined plant foods is now almost universally low in not only the developed, but also the developing world, any specific food will, therefore, in most uncontrolled studies, be compared to predominantly highly processed and animal foods (3 4). By ignoring this problem, the critics have ignored what is perhaps the most fundamental principle of nutritional research, that of dietary substitution. This problem has been addressed in detail by Walter Willett, the former chair of the Department of Nutrition at the Harvard School of Public Health, and featured expert in The Game Changers (3):
However, the study of diet is far more complicated than comparisons of pill verses placebo in randomized trials and also most other exposures evaluated in observational studies... with energy held constant, if a specific source of energy is increased, the effect on health can depend importantly on the component of diet that is reduced. In designing a randomized dietary experiment, the choice of the control diet is just as important as defining the intervention diet; the results are likely to be strongly dependent on the comparison diet.
Another focus of the critics is to downplay the certainty of evidence indicating adverse effects of animal foods, particularly from observational studies. This, to a certain extent, echoes the tobacco industry playbook of downplaying observational research as "junk science" (5 6). Indeed, the failure of randomized controlled trials to demonstrate statistically significant benefits of smoking cessation on hard-disease endpoints, including lung cancer, cardiovascular disease, and total mortality, until at least post-intervention, has ultimately helped to establish a number of underlying difficulties of using clinical trials to prove the long-term benefits of lifestyle changes (7 8 9). This is in part why all forms of evidence, including observational studies, must be evaluated to determine the effect of lifestyle changes on chronic disease and mortality.
A prominent example of how a number of important problems that commonly plague nutritional research are frequently exploited may be observed from a series of recent systematic reviews that downplayed the adverse effects of red meat on chronic disease and mortality (10 11). These reviews have come under scrutiny, including by researchers from Harvard, in part due to the use of grading criteria specifically designed to examine drug trials, in which all observational evidence is classified as low certainty by default (10). By definition, this criterion could not be used to classify even the evidence for cigarette smoking and the risk of chronic diseases, including lung cancer as high certainty, let alone that for most other environmental and lifestyle exposures, such as secondhand smoking, air pollution, and exercise. Perhaps most concerning, however, was that the review leadership failed to adequately recognize in their conclusions the critical limitation that in most studies reviewed, red meat was compared to non-specific, predominantly low-quality foods.
It should be recognized that when applying the same grading criteria commonly used to downplay the adverse effects of animal foods, it would prove difficult to justify the recommendations to restrict the intake of virtually any of the foods that the advocates of popular animal-based diets commonly call to be restricted. For example, a recent meta-analysis of prospective cohort studies found that compared to non-specific sources of energy, each 90 gm/day increment of refined grains actually associated with a statistically significant 6% and 5% decreased risk of total cancer incidence and total mortality, respectively (12). Nevertheless, the authors of this study emphasized the benefits of replacing refined grains with whole grains due to the more consistent and pronounced association with a reduced risk of chronic disease and mortality. In contrast, the leadership of the systematic reviews examining red meat recommended against reducing intake, despite their own findings from the meta-analyses of prospective cohort studies included in these reviews indicating that compared to non-specific sources of energy, each 3 serving per week increment of unprocessed red meat intake (approx. 5%E) was associated with a 12%, 8%, and 11% increased risk of cardiovascular mortality, cancer mortality, and type 2 diabetes incidence, respectively (11).
The less clear association observed for unprocessed red meat intake and total mortality in the meta-analyses of prospective cohort studies included in these reviews may in part be explained by the exclusion of relevant studies, including those the authors had classified as having a low risk of bias [Figure 1]. Evidence of an increased risk of total mortality has also been reported in multiple subsequently published studies. Therefore, for this review, an updated meta-analysis was carried out to include all relevant studies in which participants were either generally healthy, or who had been diagnosed with a cardiometabolic risk factor, but otherwise considered healthy at study baseline. Based on data involving 2 million participants with 28.4 million person-years from 30 prospective cohort studies, a high intake of unprocessed red meat was associated with a modest, but statistically significant increased risk of total mortality [Figure 1]. Importantly, the association with mortality remained statistically significant in a subgroup analysis including only those studies identified for the systematic reviews [Figure 1].
Another important limitation of the majority of epidemiological studies included in these systematic reviews was that red meat intake was only measured at study baseline, and were therefore inadequately powered to detect bias introduced by changes to intake throughout follow-up. It is well recognized that the between-person differences in exposure levels at study baseline are generally greater than the actual differences over time, leading to an underestimation of the true association (i.e. regression dilution bias). For example, it has been demonstrated in major studies that the use of baseline measurements can underestimate the true association between risk factors and disease by about one-third over the first decade, and two-thirds by the third decade of follow-up (13). This phenomenon has also been suggested to help explain the failure of several large studies to demonstrate a correlation between secondhand smoke exposure and smoking-related diseases (14). While the use of periodic repeated measurements of dietary intake was considered as part of the grading criteria in these systematic reviews, this criterion was primarily used to justify downgrading the certainty of evidence of benefit of reducing red meat intake. Notably, the authors downgraded the certainty of evidence when a “lack of periodic repeated measurement of diet” was identified for several included studies, even when benefit was observed to be more pronounced for the studies classified as having a low risk of bias. Such was the case for the analysis of reduction in unprocessed red meat intake and the risk of cardiovascular mortality (RR=0.89 [0.86-0.91] and RR=0.98 [0.80-1.19] for low and high-risk bias studies, respectively).
For the two studies the authors classified the confidence in the quantification of red meat intake as definitive, each 120 gm/day increment of unprocessed red meat intake was associated with an approximately 19%, 26%, and 14% increased risk of total, cardiovascular, and cancer mortality, respectively (15). This magnitude of risk for total mortality is actually remarkably similar to that observed for secondhand smoke exposure (16). Importantly, there was no evidence of a threshold at which a further increase in intake of red meat was not associated with a greater increased risk of total mortality [Figure 2] (15). Moreover, it was found that after accounting for measurement error resulting from dietary changes throughout 28 years of follow-up, each serving per day increment of red meat was associated with an 83% and 25% increased risk of total mortality in men and women, respectively. This increase in risk was up to 3-fold greater than that observed when only measuring baseline data, a magnitude similar to that observed in other major studies examining risk factors and disease over a similar period of follow-up [Figure 2] (13).
It was also found in these high-quality cohorts that the intake of both red meat and the associated macronutrients were associated with an even greater increased risk of total mortality when specifically substituted for appropriate sources of energy, including high-quality sources of plant protein [Figures 3-4] (17 18 19). Notably, it was found that substituting a 120 gm/day serving of unprocessed red meat for an equivalent serving of nuts over 12 years was associated with a 34% increased risk of total mortality, approximately two-fold greater than that observed for the substitution for non-specific sources of energy (17). Importantly, these findings are compatible with high-quality evidence from controlled dietary trials which have established that substituting high-quality sources of plant protein with red meat adversely affects major risk factors for cardiovascular disease and cancer [Figure 5] (20 21 22).
 |
| Figure 2. Dose-response relationship between red meat intake and relative risk of all-cause mortality in the Nurses’ Health Study and Health Professionals Follow-up Study. Red triangles indicate the effect of an increment of one serving per day of red meat intake after accounting for measurement error resulting from dietary changes throughout follow-up. From Pan et al., 2012 |
The less clear association between red meat intake and the risk of mortality in the meta-analysis of randomized controlled trials included in these reviews may also be importantly explained by the quality of foods displacing red meat. In this review, only two trials examining the effect of red meat reduction on mortality endpoints were identified, of which one, the Lyon Diet Heart Study was excluded from the primary analysis in part due to concerns of early termination bias, and because the authors considered the 56% reduction in total mortality to be implausibly high. The meta-analysis was thus limited to only the 17-year cumulative follow-up of the Women’s Health Initiative, a large trial of almost 49,000 postmenopausal women that principally investigated the effect of a low-fat diet on breast and colorectal cancer incidence. In this trial dietary changes achieved in the experimental group were far below that intended by the study design, with on average, only modest increases in intake of fruits, vegetables, and whole grains, while the intake of sugar increased by 4.8% of total energy together with an increase in intake of refined grains (23). This increase in intake of energy from refined sources of carbohydrate was approximately twice as large as the estimated 1.4 serving per-week reduction in red meat intake. Therefore, this study may better elucidate the effects of substituting red meat with refined than for unrefined sources of carbohydrate. This important limitation was not disclosed in the panel’s conclusions. Nor was there any mention of the serious bias on total and cardiovascular mortality endpoints resulting from the significantly greater use of statins in the control arm of this trial (11). John Sievenpiper, a coauthor of this meta-analysis paper who strongly disagreed with the panel’s conclusions had even specifically requested for substitution analyses to be considered, but was apparently denied this.
Unfortunately, the leadership of the paper chose to play up the low certainty of evidence by GRADE as opposed to the protective associations that directly support current recommendations to lower meat intake… The signals would be even stronger if one considered substitution analyses with plant protein sources or investigated dose-response gradients which are used to upgrade data by GRADE, both of which I had requested.
While the endpoints were not considered in this review, it was found in the Women’s Health Initiative that mortality following breast cancer and diabetes requiring insulin were both significantly lower in the experimental group during the intervention phase, benefits that persisted through cumulative follow-up. Moreover, it was found in the more recent 19.6-year cumulative follow-up that breast cancer mortality, an endpoint that was considered in this review, was reduced by 21% in the experimental group, a finding that was statistically significant [Figure 6]. While not available for the initial review process, these latter findings were documented in a report published prior to the publication of this review (24). Nevertheless, these findings were not discussed in the press release, nor does there appear to have been any attempt made by the review leadership to bring attention to these findings at a later date.
Given the exceptionally large size of the Women’s Health Initiative, the review leadership was most certainly aware of both of the principal findings of this trial and that the inclusion of this trial would inevitably dominate the weighting of the meta-analyses when planning this review. While prior knowledge of the literature does not necessarily negate the panel's conclusions, it may be necessary to consider the possibility of selection bias when interpreting the findings. Tobacco industry-linked researchers Enstrom and Kabat were subjected to universal criticism for this bias upon publishing a large meta-analysis involving over 750,000 participants that suggested a null effect of secondhand smoking on the risk of coronary heart disease mortality for including very large industry-funded studies with null findings resulting from significant exposure misclassification that dominated the weighting and obscured the estimates (14 25). Needless to state, the quality of a meta-analysis cannot be quantified by size alone.
It should also be recognized that a lead researcher of these systematic reviews was later found to have undisclosed links to the meat industry (26). While this fact alone does not necessarily negate the panel's conclusions, there is suggestive evidence indicating that the review leadership intentionally downplayed the adverse effects of red meat by downgrading the certainty of evidence even when subgroup analyses did not support the justifications for doing so, while also refusing to consider evidence requested by their coauthors that could have been used to upgrade the certainty of evidence. The leadership appears to have essentially exploited the magnitude of adverse effect observed for a limited change in intake (≤5% energy), a limited duration of exposure to intake (<10 years), and comparisons to predominantly low-quality foods to argue that red meat likely only has a small effect on the risk of chronic disease and mortality. Greatly contrasting conclusions could be drawn when specifically considering the evidence examining the long-term effects of substituting the equivalent of the 90th percentile of red meat intake for high-quality plant foods [Figure 2]. Based on the estimates from the two prospective cohort studies for which the authors classified the confidence in the quantification of red meat intake as definitive, the substitution of approximately 300 gm/day of unprocessed red meat per day for an equivalent serving of nuts, 16% of energy from unprocessed red meat protein for plant protein, or 11% of energy from saturated fat for polyunsaturated fat over ≥12 years was each associated with an approximately two-fold increased risk of total mortality, assuming a linear dose-response relationship [Figures 3-4] (17 18 19). This is a magnitude of risk comparable to that observed for cigarette smoking for an intake similar to or lower than that commonly consumed as part of some popular animal-based diets (2).
The notion that the intake of any particular food, macronutrient, or dietary pattern is an independent determinant of health is perhaps the single greatest cause of confusion surrounding diet and health. As a higher intake of one source of energy implies a lower intake of other sources of energy in order to maintain caloric balance, the effect that any food, macronutrient, or dietary pattern has on health outcomes can normally only be determined by what it is substituted for. As such, the common argument that evidence of benefit of substituting animal foods with high-quality plant foods may be related to an increase in plant food intake, and not a reduction in animal food intake lacks merit. If benefit was observed for a reduction in intake of animal foods without an equivalent increase in energy from plant foods, the critics would likely attribute this to an energy deficit, as such arguments are specifically designed to cast doubt on unfavorable evidence regardless of the findings.
In contrast to the study of most other environmental and lifestyle exposures that compare the effects of a harmful exposure to a more optimal exposure, the study of diet is often largely the opposite, as any food, macronutrient, or dietary pattern is commonly compared to among the least healthful sources of energy. Citing evidence of a lack of a substantial adverse effect of animal foods compared to other foods that dietary guidelines call to be restricted does about as little to refute these guidelines as the evidence of a lack of an adverse effect of menthol compared to non-menthol cigarette smoking does to refute recommendations to avoid any form of cigarette smoking. Rather, evidence of lack of benefit compared to other suboptimal foods may be considered evidence of harm. In order to determine both the true magnitude of effect of diet, and which foods should be consumed for optimal health, it is of critical importance to compare foods to healthy alternatives, not just to the most commonly consumed foods as is typically done. To understand diet and health, we should compare apples not to cakes, cookies, and cream, but to oranges.
Not Settling for Mediocrity
As cardiovascular disease accounts for approximately one-third of all global deaths, it merits strong emphasis that controlled feeding trials have established that a high-quality plant-based diet can reduce apolipoprotein B (apo-B) and low-density lipoprotein cholesterol (LDL-C) by 25 mg/dl and 1 mmol/l, respectively, over and above that achievable with therapeutic diets commonly recommended by major health authorities, predicting a greater than 50% reduction in the lifetime risk of major cardiovascular events [Figure 7] (27 28 29 30 31 32 33 34). The sheer quantity and quality of data simply leaves no room for any other interpretation. Evidence from over 1,000 controlled dietary experiments has unequivocally established that substituting animal foods, particularly those rich in saturated fat with high-quality plant foods lowers the concentration of apo-B-containing lipoproteins, including LDL and, in turn, evidence from over 200 clinical and genetic studies involving over 2 million participants and more than 200,000 cardiovascular events has unequivocally established that lowering the concentration of apo-B-containing lipoproteins reduces the risk of cardiovascular disease [Figures 5, 7-15] (31 32 33 34 35 36 37 38 39 40 41 42).
 |
| Figure 12. 112 experiments of plant protein for animal protein and changes to LDL-C (mmol/l), non-HDL cholesterol (mmol/l), and apolipoprotein B (g/l). From Mejia et al., 2017 |
 |
| Figure 13. 182 experiments of plant sterol and stanol intake and percentage change to LDL-C. From Musa-Veloso et al., 2011 |
The Game Changers documents a 7-day whole-foods plant-based dietary challenge conducted by Rip Esselstyn that demonstrated significant improvements to several disease risk factors, including a 21 mg/dl mean and up to 107 mg/dl reduction in total cholesterol, and a 2.8 kg mean reduction in body weight. The critics commonly argue that the evidence of benefit of a plant-based diet, such as those described in the documentary can be explained almost entirely by comparisons with suboptimal diets rich in processed foods, and that these benefits would not be replicated when compared to an unprocessed diet richer in animal foods. While such criticism may be welcome, it makes it abundantly clear that the critics are aware both of the importance of dietary substitution, and that many of the studies that they themselves cite to argue against restricting the intake of animal foods are based on comparisons with the same suboptimal sources of energy that they commonly argue are major causes of chronic disease. Moreover, such criticism ignores a substantial body of evidence from controlled feeding experiments indicating that the magnitude of efficacy of a high-quality plant-based diet for reducing disease risk factors is so great, that it is likely comparable to that of combining multiple powerful pharmaceutical agents, both independent of weight loss and over and above that achievable with therapeutic diets commonly recommended by major health authorities.
David Jenkins, known for developing the glycemic index, carried out a number of controlled feeding trials comparing a weight-maintaining plant-based Portfolio diet, focusing on the combined effects of various cholesterol-lowering plant foods with the National Cholesterol Education Program (NCEP) Step II diet. These experiments demonstrated that various plant foods have an additive effect on reducing apo-B and LDL, including both large buoyant and small dense LDL particles, resulting in an absolute reduction comparable to the efficacy of statin therapy [Figure 15] (28 29). Jenkins has also demonstrated that compared to an NCEP Step II diet, a weight-maintaining, whole-foods plant-based Simian diet, predominated by foods such as fruit, vegetables, and nuts consumed during the Miocene period similarly reduced apo-B by about 24 mg/dl, while also improving measures of colonic function [Figure 15] (30).
A substantial body of evidence indicates that a high-quality plant-based diet can also significantly reduce blood pressure (43). Meta-analyses of randomized controlled trials have demonstrated that when consumed in quantities comparable to that commonly consumed as part of a whole-foods plant-based dietary pattern, the intake of a number of nutrients almost exclusively derived from plants, including dietary fiber, vitamin C, and polyphenols can each reduce systolic blood pressure (SBP) by between about 1.3 and 6 mmHg (44 45 46 47). Additive, this may result in a reduction in SBP of up to more than 10 mmHg, an effect comparable to the efficacy of common blood pressure lowering medications. Indeed, a controlled feeding experiment found that a raw whole-foods plant-based diet reduced SBP by 16.6 mmHg in hypertensive patients after 4 weeks compared to baseline diet (48). Similarly, a pooled analysis of 4 controlled feeding experiments found that a low-fat whole-foods plant-based diet reduced SBP by 10 mmHg after 21 days compared to baseline diet (49). Most recently, a controlled feeding trial found that a minimally refined low-fat plant-based diet reduced diastolic and systolic blood pressure by about 2 and 4 mmHg, respectively, after two weeks compared to a minimally processed animal-based, but otherwise vegetable-rich ketogenic diet (50).
The benefit of a whole-foods plant-based dietary pattern on reducing blood pressure is also supported by a body of epidemiological evidence (43). In one cross-sectional study, it was found that SBP was 18 mmHg lower among sedentary raw vegans compared to BMI-matched omnivorous runners (43). This magnitude of reduction is of critical importance, especially given the evidence that the effect of lowering blood pressure on reducing the risk of cardiovascular disease is multiplicative with other cardiovascular risk factors. In a recent Mendelian randomization study involving over 438,000 participants, it was found that a lifetime of cumulative exposure to a combined 30 mg/dl lower apo-B and 10 mmHg lower SBP was causally associated with an 80% reduced odds of major coronary events (33).
Evidence also indicates that the magnitude of benefit of a whole-foods plant-based diet on reducing blood glucose may be comparable to the efficacy of diabetic medication, both independent of weight-loss, and over and above that achievable with diabetic diets commonly recommended by major health authorities. It was found in a controlled feeding trial that in addition to reducing LDL-C by 0.65 mmol/l, a whole-foods Ma-Pi 2 macrobiotic diet with 73% of energy from carbohydrate significantly improved blood glucose levels compared to a calorie-matched minimally processed Mediterranean diet commonly recommended to diabetic patients (51). Importantly, all the participants on the Ma-Pi 2 diet achieved target blood glucose levels, and on average achieved non-diabetic fasting blood glucose levels (<100 mg/dl) [Figure 16]. Similarly, in a pooled analysis of 4 earlier controlled feeding trials, it was found that in addition to reducing LDL-C by 1.03 mmol/l and SBP by 10 mmHg compared to baseline diet, a Ma-Pi 2 diet with 70% of energy from carbohydrate resulted in a 50% reduction in insulin consumption after only 21 days, with an 82% reduction in the most tightly controlled experiment (49). In another controlled feeding trial dating back four decades, it was found that in addition to reducing total cholesterol by 1.53 mmol/l, a weight-maintaining whole-foods plant-based diet with 70% of energy from carbohydrates resulted in a 58% reduction in insulin consumption after an average of only 16 days compared to the traditionally recommended diabetic diet (52). Moreover, insulin consumption was discontinued altogether in 9 of 10 patients initially receiving 15 to 20 units/day and in two patients receiving 32 units/day [Figure 17].
The critics often argue, citing the carbohydrate-insulin model of weight loss, that substituting carbohydrate-containing plant foods with fat-rich animal foods results in fat loss and, in turn, attenuates the adverse effects of a diet rich in animal foods. However, a substantial body of high-quality evidence has strongly refuted the carbohydrate-insulin model of weight loss. Most notably, a meta-analysis of 32 controlled feeding trials of isocaloric substitution of carbohydrate for fat, with total energy ranging from 1% to 83% from carbohydrate, and 4% to 84% from fat, found that both energy expenditure and fat loss were slightly greater with the lower fat diets [Figure 18] (53). Moreover, a separate controlled feeding trial funded by the Nutrition Sciences Initiative (NuSi), which promotes ketogenic diets, similarly found that body fat loss slowed while participants were adhering to a ketogenic diet compared to an isocaloric high-carbohydrate diet containing <2% and 25% of energy from sugar, respectively (54). Most recently, a controlled feeding trial carried out by the same study group found a 35 gm/day greater body fat loss and a 689 kcal/day greater reduction in energy intake over two weeks while participants were adhering to an ad libitum minimally processed plant-based diet with a high glycemic load compared to an ad libitum minimally processed animal-based ketogenic diet, containing 75% and 10% of energy from carbohydrate, and 10% and 75% of energy from fat, respectively (50).
Causal evidence examining the long-term effect of diet also casts significant doubt on the carbohydrate-insulin model of weight loss. A recent Cochrane review involving 57,079 participants from 37 randomized controlled trials lasting at least six months, and with no intention of inducing weight loss, found that lower compared to higher fat intake resulted in lower body weight, BMI, waist circumference, and percentage body fat, and that greater reductions in fat intake associated with greater reductions in body weight (55). Moreover, a recent Mendelian randomization study based on dietary data from over 268,000 participants and more than 232,000 anthropometric measurements found that a genetically predicted lower relative intake of carbohydrate, a higher intake of fat, and possibly a higher intake of protein as a percentage of total energy was causally associated with both a higher BMI and higher waist circumference [Figure 19] (56). Given the lifelong nature of Mendelian randomization studies, this indicates that the effect of dietary fat on weight gain is likely to be sustained permanently.
Evidence from controlled feeding experiments in animals, including nonhuman primates has also consistently demonstrated that high-fat low-carbohydrate diets are more effective at inducing obesity than low-fat high-carbohydrate, even when energy intake is held constant (57). In a recent feeding experiment on 5 strains of mice using 29 diets with total energy varying between 8.3% to 80% fat, 10% to 80% carbohydrate, 5% to 30% protein, and 5% to 30% sucrose, it was found that only an increase in dietary fat associated with either increased energy intake or body fat (58).
While some longer-term randomized controlled trials that lacked a controlled feeding design have produced contrasting conclusions regarding weight loss, these studies are comparably less suitable for demonstrating efficacy. It is well documented that when prescribed low-fat diets, as with many diets, free-living study participants consistently under-report energy intake and over-report adherence (59). It has also been consistently found that when prescribed the same diet, free-living study participants achieve only about 50% of the changes to blood cholesterol levels predicted by that from highly controlled feeding experiments, further demonstrating this problem of non-adherence (28 60).
It is important to recognize that because 1 kg (2.2 lb) of body fat stores 7,700 kcal of energy, each decrease of 21 kcal/day, or the equivalent of a single macadamia nut can explain all the variance of a 1 kg fat loss in an intervention study lasting one year. This indicates sufficient room to exploit long-term trials to falsely conclude that certain diets, such as a low-carbohydrate diet can reduce body fat independent of energy intake. If researchers, even unconsciously, motivate the participants in the experimental group enough to consume marginally fewer calories per day relative to the controlled group, it is likely that comparatively, the experimental group would experience a statistically significant increase in long-term fat loss, despite no statistically significant differences in daily caloric intake. These concerns are among a number of reasons why controlled feeding trials, particularly those in which all food is provided to help minimize confounding by non-adherence, are more suitable for studying the effect of diet on body fat (53).
Multiple other lines of evidence examining the effect of fiber-rich diets also indicate that a whole-foods plant-based dietary pattern is optimal for reducing body fat. A recent systematic review commissioned by the World Health Organization found based on evidence from clinical trials that in addition to reducing systolic blood pressure, fasting glucose, triglycerides, and total and LDL cholesterol, dietary fiber also reduced body weight [Figure 20] (44). Similarly, a recent meta-analysis of placebo-based clinical trials without energy-restriction protocols found that in addition to reducing fasting glucose and fasting insulin, soluble fiber also reduced BMI, body weight, and body fat (61). Direct evidence from both randomized controlled trials and large prospective cohort studies also indicates that a fiber-rich plant-based dietary pattern can reduce body fat (62 63).
 |
| Figure 20. Effect of total dietary fiber intake on body weight and cardiovascular risk factors in a meta-analysis of randomized controlled trials. From Reynolds et al., 2019 |
Carefully conducted controlled feeding experiments have consistently demonstrated that the magnitude of efficacy of a high-quality plant-based diet for reducing disease risk factors is actually comparable to that of combining multiple powerful pharmaceutical agents, both independent of weight loss and over and above that achievable with therapeutic diets commonly recommended by major health authorities [Figures 15-17]. In turn, these therapeutic diets are predicted to result in a significant residual risk of chronic disease compared to a more focused high-quality plant-based diet, and are at best mediocre in terms of health. Importantly, many of the described benefits of a high-quality plant-based, including a 25 mg/dl reduction in apo-B, are for direct comparisons with commonly recommended therapeutic diets, and would be far greater compared to popular diets much richer in animal foods, as can be extrapolated from the findings from over 1,000 controlled dietary experiments [Figures 5, 8-18, 20]. In addition, a substantial body of high-quality evidence from controlled feeding experiments, long-term randomized controlled trials, and Mendelian randomization of the short, long-term, and permanent effects of dietary fat on weight gain strongly refute the validity of the carbohydrate-insulin model of weight loss, often argued as a primary benefit of popular animal-based diets [Figures 18-19]. While the available evidence favors the energy balance model over the carbohydrate-insulin model of weight loss, it also indicates room for meaningful improvement through a greater focus on minimally refined fiber-rich plant-based diets, which may reduce body fat in part independent of energy intake [Figure 20]. The observation that obesity rates remain high in many parts of the world in itself does not necessarily negate the efficacy of the energy balance model in light of the evidence of low rates of compliance. While problems with compliance can evidently be explained by multiple social and economic factors, it is difficult to rule out the influence of the multi-decade-long misinformation campaign of the critics dating back at least half a century ago with the advent of the Atkins diet.
Randomized at Birth
It has been unequivocally established that the lifetime risk of developing atherosclerotic cardiovascular disease is importantly determined by both the magnitude and cumulative duration of exposure to apolipoprotein B (apo-B)-containing lipoproteins as the result of retention and accumulation of these particles in the artery wall over time (31 32). Over 40 years ago, Sir Richard Peto, known for his contribution to the development of the meta-analysis, demonstrated for the first time in a meta-analysis of randomized controlled trials, a causal dose-dependent effect of blood cholesterol levels on the risk of coronary heart disease (64). It has, however, only been established within the last decade, primarily with the contribution of large Mendelian randomization studies, that the cumulative duration of exposure to apo-B-containing lipoproteins, including LDL, also substantially increases the risk of cardiovascular disease. Mendelian randomization studies, while not without limitations, have been considered a proxy to a lifelong randomized controlled trial, as the measured variation in genes that affect modifiable exposures, such as blood lipids, are allocated approximately randomly at the point of conception (65). Mendelian randomization studies have recently been able to help fill a number of important evidence gaps that would have otherwise been impossible or impractical to conduct as a standard randomized controlled trial. In particular, these studies have helped to establish that compared to 5 years of lower exposure later in life, a lifetime of exposure to lower LDL cholesterol results in a 3-fold greater reduction in risk of cardiovascular disease, proportional to the absolute reduction in LDL-C [Figure 7] (31 32). Moreover, these studies have helped to establish that lowering LDL-C reduces the risk of total mortality, even in very advanced age (>90 years), and that simply raising the concentration of apolipoprotein A-I-containing lipoproteins, including HDL-C does not translate into a reduced risk of cardiovascular disease or total mortality (66 67 68 69 70).
The causal relationship between LDL-C and cardiovascular disease has in part been established by the remarkably similar effect proportional to the absolute reduction in LDL-C between multiple cholesterol-lowering therapies observed in randomized controlled trials, and between each genetic score from variants in over 50 genes observed in Mendelian randomization studies (31). Moreover, the variants in genes that encode the targets of cholesterol-lowering therapies, including diet, also have a remarkably similar effect as the actual cholesterol-lowering therapies, proportional to both the absolute reduction in LDL-C and cumulative duration of exposure to lower LDL-C [Figure 21-23] (31 32 71 72).
A meta-regression analysis of 49 randomized controlled trials involving 312,000 participants found that 8 separate LDL-C lowering therapies, including diet, were each associated with a remarkably similar reduction in major vascular events proportional to the absolute reduction in LDL-C [Figure 22] (72). Overall, each 1 mmol/l reduction in LDL-C over a median follow-up of 4.3 years was associated with a 23% reduction in risk of major vascular events. The only notable exception to these findings was the lack of benefit observed for CETP inhibitors. However, since publication, another trial involving over 30,000 participants with 4 years of follow-up found that statin plus CETP inhibitor significantly reduced major coronary events compared to statin plus placebo, and that this effect was proportional to that predicted by the absolute reduction in non-HDL cholesterol (73). This indicates that the findings from earlier trials that failed to reduce events may either be explained by deleterious off-target effects of other CETP inhibitor drug types, or perhaps more likely, a consequence of a much shorter treatment period.
Several recent pooled analyses of Mendelian randomization studies collectively involving over 700,000 participants found that lower LDL-C was causally associated with a reduced risk of total mortality, with no significant benefits observed for higher HDL-C after controlling for LDL-C (69 70). Consistent with these findings, a meta-regression analysis of 108 randomized controlled trials of lipid-modifying therapies involving a further 300,000 participants found that after controlling for LDL-C, no benefit of increasing HDL-C on coronary heart disease events, deaths, or total deaths. Conversely, each 1 mmol/l reduction in LDL-C over a median follow-up of 3 years was associated with a 16% and 25% reduction in risk of total and coronary heart disease mortality, independent of other blood lipids and non-lipid effects of specific interventions (74).
A recent meta-analysis of randomized controlled trials also found a similar reduction in risk of major cardiovascular events proportional to the absolute reduction in LDL-C in participants both under and over the age of 75 (75). Moreover, another recent meta-analysis of randomized controlled trials found a similar reduction in risk of major cardiovascular events in participants with low baseline LDL-C, and without evidence of a threshold at which further reduction did not provide greater benefit, down to at least 0.5 mmol/l (21 mg/dl), and without any major adverse effects [Figure 24] (76). These low achieved LDL-C levels are actually similar to that observed in both newborns and free-ranging nonhuman primates, and as such, may be considered as intrinsic or natural levels. Additionally, individuals who maintain levels as low as 1 mg/dl throughout life due to rare genetic disorders have been observed to experience normal growth without notable adverse effects (77 78 79).
 |
Figure 24.
|
The critics commonly argue that small dense LDL particles are the primary lipid determinant that explains the relationship between LDL and atherosclerotic cardiovascular disease, often citing observational evidence in which small dense LDL particles associated with cardiovascular disease independent of LDL-C. This plays on the argument that animal-based nutrients, including saturated fat, increase predominantly large buoyant LDL particles (21). However, clinical trials have found that small LDL particles are also reduced when saturated fat is substituted with either polyunsaturated or monounsaturated, and when consuming foods rich in dietary fiber and phytonutrients (4 29). Thus, restricting the intake of animal foods in favor of high-quality plant foods is likely a useful strategy for reducing the concentration of small LDL particles. Nevertheless, a substantial body of evidence indicates that all apo-B-containing lipoproteins, including large LDL, similarly contribute to the risk of cardiovascular disease. Notably, many studies have found that the independent association observed between small dense LDL particles and cardiovascular disease is significantly attenuated or abolished when adjusted for apo-B and other risk factors (80). Large LDL particles predominate in individuals with familial hypercholesterolemia who are at risk of very premature cardiovascular events (80). Individuals who inherit homozygous familial hypercholesterolemia, a very rare form of this disorder characterized by total cholesterol levels of >13 mmol/l (>500 mg/dl), rarely survive past 30 when left untreated, and often experience major cardiovascular events even in early childhood (81).
Recently, several pooled analyses of Mendelian randomization studies collectively involving 1 million participants and more than 170,000 events found that only apo-B retained a robust effect on coronary heart disease and ischemic stroke after mutual adjustment for LDL-C, triglycerides, HDL-C, and apolipoprotein A-I (67 68 82 83). Moreover, an analysis involving over 453,000 participants and 114,000 coronary events which considered 30 lipoprotein measures and metabolites, including 14 size categories of lipoprotein particles, both alone and in combination, found that apo-B by itself was the best predictor of risk [Table 1] (68). Separately, a recent meta-analysis of 29 randomized controlled trials involving a further 333,000 participants and more than 35,000 events, found that lowering apo-B reduced the risk of major adverse cardiovascular events in part independent of changes to LDL-C and triglycerides (34). Similar findings were also observed in a recent prospective cohort study involving 430,000 participants with 4.4 million person-years of follow-up (84). Thus, the totality of evidence from clinical, genetic, and epidemiological studies strongly indicates that apo-B, representing the total number of atherogenic particles is the primary lipid determinant of atherosclerotic cardiovascular disease, with little or no benefit of modifying the size of lipoprotein particles, or raising HDL, independent of apo-B.
It is important to recognize that in the pre-statin and early statin eras, diet explained most of the observed reduction in cholesterol levels in industrialized nations, in part as the result of Government policies that emphasized dietary change (85). Risk factor models have consistently demonstrated that changes to cholesterol levels explain a significant portion of both the major declines in coronary heart disease mortality observed in industrialized nations throughout the last half-century, as well as the major increases in a number of developing nations (85 86 87). In a number of former communist nations of Eastern Europe, there was a steady rise in coronary heart disease mortality observed during the 1970s and 1980s, followed by a very significant decline in the 1990s after the communist subsidies on meat and animal fats were abolished with the breakup of the Soviet Union (88 89 90). Within 15 years heart disease mortality was reduced by up to 50%, being among the fastest declines observed in the world [Figures 25-26] (88 89 90 91).
Finland, which by the 1960s had the highest rate of coronary heart disease mortality in the world, experienced a greater than 80% decline in both men and women following successful government initiatives. This decline was predominantly explained by a significant reduction in cholesterol levels following improvements to the quality of dietary fat, particularly through a large reduction in dairy fat, as well as increases in fruit and vegetable intake, and was despite a doubling of smoking prevalence among women over the same period [Figure 28] (92 93 94). Reductions in cholesterol levels have also been observed as the main contributor to the significant decline in heart disease mortality observed in other Scandinavian nations, which decreased by at least 50% within three decades following similar government initiatives (95 96). In contrast, in Beijing where the rate of coronary heart disease mortality increased by more than 100% between 1984 and 1999, the great majority of the increase was explained by an increase in cholesterol levels following a five-fold increase in meat and egg intake (97).
 |
Figure 25:
|
 |
| Figure 27. Observed and predicted declines in mortality from coronary disease in men (A) and women (B) aged 35-64 in eastern Finland. From Jousilahti et al., 2016 |
Evidence from over 200 clinical and genetic studies indicating that at least 9 separate LDL-lowering therapies, which each modify LDL by distinct biological pathways, all have remarkably similar effects on reducing the risk of major cardiovascular events proportional to both the magnitude and cumulative duration of exposure to LDL has unequivocally established that LDL particles cause atherosclerotic cardiovascular disease [Figures 7, 21-24]. In addition, separate evidence from over 100 clinical and genetic studies involving over 1.3 million participants and more than 200,000 major cardiovascular events has established that apolipoprotein B (apo-B) is the primary lipid determinant of atherosclerotic cardiovascular disease, with little or no benefit of modifying the size of lipoprotein particles, or raising HDL, independent of apo-B (34 67 68 83). Apo-B, not the size or subclass is what primarily exerts the atherogenic effect of lipoprotein particles.
Evidence from over 130 clinical and genetic studies involving over 1 million participants and more than 75,000 deaths has also established that lowering LDL reduces the risk of total mortality, with little or no benefit of modifying HDL (69 70 73). In addition, a recent Mendelian randomization study involving over 1 million participants found that 42% of the causal effect of LDL-C on premature death was independent of coronary heart disease and ischemic stroke, indicating the adverse effects of higher LDL-C extend beyond atherosclerotic cardiovascular disease. Importantly, the adverse effect was found to be largely independent of the mechanism in which LDL was modified, indicating that lifestyle changes, including diet that lower LDL also improves longevity (98). Moreover, another recent Mendelian randomization study found that after mutual adjustment for LDL-C and triglycerides, only apo-B retained a robust effect on lifespan, indicating that as with atherosclerotic cardiovascular disease, all atherogenic particles regardless of size and subclass similarly contribute to the risk of premature death (99).
While Mendelian randomization studies examining the direct effect of diet can meaningfully contribute to the understanding of diet and health, like all studies on diet, these will need to be interpreted with caution in the absence of a suitable comparison diet. This is because many genetic variants associated with diet will only inform the investigator that the intake of a particular source of energy has been increased, and not what it has displaced. If the recruited population consumes a universally low-quality diet, a Mendelian randomization study would be susceptible to the same problem as most uncontrolled studies in which any comparison will be in the context of a low-quality diet.
The totality of evidence strongly indicates that because changes to diet have an additive effect on lowering apo-B-containing lipoproteins, and the threshold of benefit of lower apo-B is lower than what can normally be achieved by lifestyle changes alone, there is no null point at which a further reduction in intake of animal foods in favor of an increased intake of high-quality plant foods would not further reduce the risk of cardiovascular disease in the general population. To claim otherwise would suggest the existence of a very substantial body of high-quality evidence that the non-lipid benefits of increasing the intake of animal foods outweigh the adverse effects of a higher apo-B, in addition to all other established non-lipid benefits of high-quality plant foods. The fact that the critics apparently so often feel the necessity to cast doubt on the established effects of apo-B-containing lipoproteins, indicates, if anything, a lack of substantial evidence to support such a narrative.
Ignoring a Century of Atherosclerosis Research
It has been established beyond reasonable doubt that a high-quality plant-based diet can significantly improve multiple major cardiovascular risk factors, including apolipoprotein B (apo-B). More importantly, a substantial body of high-quality evidence from over a century of research has established that a plant-based diet not only reduces the risk of major cardiovascular events but also likely promotes the reversal of atherosclerotic plaque formation. Dr. Dean Ornish and Dr. Caldwell Esselstyn, two of the featured experts in The Game Changers have published studies that have both supplemented and further advanced the knowledge of reversing atherosclerosis through dietary means (100 101 102 103 104).
In The Lifestyle Heart Trial carried out by Dr. Dean Ornish, it was found that participants in the lifestyle change group, which included a plant-based diet, very rich in complex carbohydrates, reduced apo-B and LDL-C by 29 mg/dl and 1.43 mmol/l, respectively, compared to the usual-care control group, achieving an absolute LDL-C of 2.24 mmol/l (87 mg/dl) after 1 year. Regression of atherosclerotic plaque was demonstrated in the lifestyle group after only 1 year, with further regression after 5 years [Figure 28]. Moreover, compared to the control group, the lifestyle group experienced 60% fewer cardiac events and a 91% reduced frequency of angina (104). While it is not possible to entirely rule out the benefits of other lifestyle changes made by the experimental group, including moderately higher aerobic exercise levels, stress management, and psychosocial support, it should be recognized that in an earlier controlled feeding trial, Ornish demonstrated that a strict plant-based diet and stress management alone similarly reduced the frequency of angina by 91% (104 105).
In a dietary intervention study carried out by Dr. Caldwell Esselstyn, it was observed that of the 177 (89%) high-risk patients who adhered to a whole-foods plant-based diet, very rich in complex carbohydrates, only 0.6% experienced a recurrent major cardiac event rate, none of which were fatal (100 101 102 103). Similar findings were observed in an earlier 12-year follow-up of a smaller group of 18 adherent patients (101). Esselstyn has also demonstrated a very remarkable degree of regression of atherosclerosis in a number of patients adhering to a plant-based diet, including in those who abstained from using cholesterol-lowering medications [Figure 29].
 |
Figure 28. Mean percentage diameter stenosis in treatment and control groups at baseline, 1 year, and 5 years in The Lifestyle Heart Trial. From Ornish et al., 1998
|
The critics commonly argue that the benefits observed in the studies by Ornish and Esselstyn are unlikely explained by the substitution of animal foods with high-quality plant foods, but rather by other lifestyle changes and a reduction in intake of processed foods. These arguments, however, not only largely ignore the evidence from over 200 clinical and genetic studies involving 2 million participants that has unequivocally established that apo-B-containing lipoproteins cause atherosclerotic cardiovascular disease, but also ignore thousands of experimental atherosclerosis studies in over 100 species and breeds of animals from over the last century (31 32 67 68 72 74 77).
The change in progression and regression of atherosclerosis across clinical trials of cholesterol-lowering therapies has consistently been found to be largely proportional to the absolute achieved LDL-C and apo-B [Figures 30] (31 106 107 108). Clinical trials have also established that regression of atherosclerosis is on average achieved when LDL-C is reduced to below about 1.8 mmol/l (70 mg/dl), without evidence of a threshold at which a further reduction does not provide greater benefit, down to at least 0.5 mmol/l (20 mg/dl) [Figures 30-31]. These findings are compatible with the evidence from Mendelian randomization studies indicating that higher LDL-C, but not lower HDL-C promotes the progression of atherosclerosis (109 110).
 |
| Figure 31. Change in atherosclerosis as measured by intravascular ultrasound compared to absolute achieved LDL cholesterol as observed in the GLAGOV clinical trial. From Nicholls et al., 2016 |
Since the breakthrough led by Nikolai Anichkov more than a century ago, the feeding of dietary cholesterol has virtually been recognized as the sine qua non for the dietary modification of experimental atherosclerosis, and has been used in thousands of experiments to successfully accelerate the development of atherosclerosis in over 100 species and breeds of mammals, birds, and fish of herbivorous, omnivorous, and carnivorous nature [Table 2] (64 77 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127). Indeed, carnivorous animals have been found to develop atherosclerosis in response to cholesterol feeding under certain conditions, including when challenged with very large intakes, or when found to be genetically susceptible, or made susceptible to a diet-induced increase in apo-B-containing lipoproteins [Table 2]. Importantly, it has been demonstrated that the feeding of fresh egg yolk has a similar adverse effect on plaque formation compared to powdered egg yolk, indicating the adverse effects of cholesterol feeding are at least in part independent of oxidation products (125). Daniel Steinberg, one of the pioneers of research on blood cholesterol has summarized this vast body of research (64).
The point is very clearly made: the arteries of virtually every animal species are susceptible to this disease if only the blood cholesterol level can be raised enough and maintained high enough for a long enough period of time.
Most relevant to the study of humans, experimental atherosclerosis has also been induced in over 20 species of nonhuman primates, including the chimpanzee, either in part, or exclusively by the feeding of dietary cholesterol [Figure 32, Table 3] (114 115 116 117 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144). Saturated fat, both from animal sources and coconut oil has also been extensively used to accelerate the development of atherosclerosis and induce heart attacks, often in comparison to refined sources of carbohydrate and omega-6-rich vegetable oils [Figure 32] (114 138 142 143 145). Experimental atherosclerosis has also been induced in primates even when comparatively, the atherogenic diet increased both HDL cholesterol and predominantly large buoyant LDL particles, of which the latter, in many cases, was found to be a better predictor of atherosclerosis than small dense LDL particles (142 146). Moreover, prolonged exposure of primates to diets rich in cholesterol and saturated fat has even been found to promote atherosclerotic plaque formation in the pelvic and penile arteries, causing erectile dysfunction (147 148).
f
In one notable experiment, it was found that compared to a cholesterol-free diet, atherosclerosis was induced in primates by the feeding of very small amounts of cholesterol over the period of only 18 months, independent of differences to LDL-C [Table 4]. Given the short duration of this experiment comparative to the average lifespan of a human living in the industrialized world, these findings indicate that even if this primate model exaggerates the atherogenic effect of dietary cholesterol in humans by a magnitude of 100, the equivalent intake of cholesterol from one small egg per day over the period of a lifetime would still significantly contribute to the development of atherosclerosis. Interestingly, these findings actually contrasted the expectations of the researchers who designed this experiment (130):
The regimen for group 1 [fed the equivalent of cholesterol from half a small egg/day in a human diet of 2,000 kcal] was originally designed to demonstrate a null point of the effect of dietary cholesterol on the arterial intima. However, such a point was not found; no threshold for dietary cholesterol was established with respect to a putatively adverse effect on arteries.
 |
| Table 3. Select species of nonhuman primates found to develop atherosclerosis in response to feeding of dietary cholesterol, either together with or without sources of saturated fat |
The evidence that a diet rich in cholesterol and saturated fat causes the development of atherosclerosis in primates is at least as, if not even more consistent than for tobacco smoke exposure. As is the case for most experiments examining non-lipid-modifying exposures, cigarette smoke exposure has most often been used not to induce, but rather aggravate the acceleration of atherosclerosis induced by a diet rich in cholesterol (149 150 151). This is consistent with the clinical evidence that most non-lipid risk factors are primarily exacerbating factors, as opposed to causative agents of atherosclerosis (152).
Atherosclerosis regression experiments in laboratory animals date back almost a century to when Anichkov initially experimented with removing dietary cholesterol used to induce atherosclerosis from the diets of rabbits. More relevant to humans, however, regression experiments in nonhuman primates dating back more than half a century have consistently demonstrated that the cessation of an atherogenic diet can promote the regression of atherosclerosis, generally so long as blood cholesterol levels are reduced low enough for a long enough period of time [Table 5, Figures 33-34] (153 154 155). Consistent with the findings from clinical trials in humans, the greatest improvements were generally observed when total cholesterol levels were lowered to below 150 mg/dl, with limited or no benefit observed at higher levels typical in experiments where cholesterol intake was maintained at levels considered more realistic to Western populations (155 156). In a number of experiments, significant regression was observed even when the sources of dietary cholesterol and saturated fat used to induce atherosclerosis, including egg yolk, butter, beef tallow, and coconut oil, were substituted with very large quantities of refined sugar and omega-6-rich vegetable oils [Table 5, Figure 33] (157 158).
The substitution of animal protein with plant protein, particularly soy protein has also been found to reduce the progression of atherosclerotic plaque size in nonhuman primates by up to 90% [Figure 35] (159). These findings have been replicated in numerous experiments and have been explained by improvements to both LDL-C and non-lipid risk factors (159 160 161 162 163). A high intake of alfalfa meal has also been found to reduce the progression of atherosclerosis in nonhuman primates independent of changes to total cholesterol levels, indicating a benefit of minimally processed plant foods, rich in fiber and antioxidant phytochemicals, both independent of and in addition to changes to traditional risk factors (155).
 |
| Figure 34. Regression of atherosclerosis in cynomolgus monkeys on a low-fat, cholesterol-free diet after atherosclerosis was induced by a diet with 40% of energy from egg yolks. From Armstrong, 1976 |
The remarkably consistent findings from clinical trials of cholesterol-lowering therapies and animal models of experimental atherosclerosis provides confidence that the substitution of animal foods with cholesterol-lowering plant foods can explain a substantial degree of the regression of atherosclerosis observed in the studies by Ornish and Esselstyn, while not necessarily negating evidence of further benefit of substituting refined with minimally refined plant foods. Importantly, participants adhering to a high-quality plant-based diet in these studies experienced both a reduction in major cardiovascular events and regression in atherosclerosis greater than that predicted by changes to blood lipids alone. Perhaps most strikingly, however, was the finding from the larger intervention study by Esselstyn of a lower residual risk of major cardiovascular events among adherent patients than in any other major intervention study of pharmaceutical agents or dietary changes in patients with preexisting cardiovascular disease (100 101 102 103).
It is important to recognize that while clinical trials have established that cholesterol-lowering pharmaceutical agents can significantly reduce the risk of cardiovascular disease, the benefits can almost entirely be explained by reductions in apo-B, and still result in a significant residual risk of cardiovascular disease [Figure 7, 21-23]. These pharmaceutical agents also often have major deleterious off-target effects, including an increased risk of developing type 2 diabetes (78). While highly processed cholesterol-lowering diets have also been found to reduce the risk of cardiovascular disease in the background of a Western-type diet by a magnitude comparable to statin therapy, these benefits too can be almost entirely be explained by reductions in apo-B, resulting in a similar residual risk of cardiovascular disease [Figures 21-22, 36-37] (4 164).
Causal evidence of a direct benefit of a cholesterol-lowering diet is indicated by a recent Cochrane review of randomized controlled trials which found that reducing the intake of saturated fat, displaced by either polyunsaturated fat or carbohydrate, predominantly from refined sources, reduced the risk of combined cardiovascular events by between about 17% and 21% after a mean follow-up of 4.4 years (164). Importantly, it was found in a meta-regression analysis that the absolute change to total cholesterol levels accounted for 99% of the between-study variation, indicating that benefit was mediated largely by changes to blood lipids (164). Estimates were however not provided for the effect of substitution with individual macronutrients relative to changes to blood lipids. Therefore, for this review, a meta-regression analysis was carried out based on data available from the Cochrane review to examine this effect. Based on 10 randomized controlled trials involving over 53,000 participants and more than 4,500 cardiovascular events, a 1 mmol/l reduction in total cholesterol primarily through a reduction in saturated fat intake was associated with a 33% reduction in cardiovascular events, with at least a similar magnitude of effect observed for trials that increased intake of either polyunsaturated fat or carbohydrate (RR=0.67 [0.53-0.84] using the random-effects model) [Figure 36]. Importantly, a similar magnitude of effect was observed when restricting the analysis to only the 4 most recent trials, casting doubt on the argument that benefits observed in the earliest trials may be primarily explained by a reduction in intake of trans-fat which was ubiquitous in the food chain at the time [Figure 36]. Moreover, given the evidence that a lifetime of cumulative exposure to lower apo-B-containing lipoproteins results in about a 3-fold greater reduction in risk compared to 5 years of cumulative exposure later in life, this indicates that even if these estimates exaggerate the true effect by 50%, a lifetime of exposure to a low intake of saturated fat may still reduce the risk of cardiovascular disease by up to more than 50% [Figures 7, 21]. While a substantial reduction in risk, this nevertheless still indicates a residual risk of up to about 50%.
Causal evidence of a direct benefit of a cholesterol-lowering diet is also indicated by the findings from recent Mendelian randomization studies examining the effect of circulating levels of essential polyunsaturated fatty acids, fatty acids that cannot be synthesized in the body, and obtained only in the form of diet. In a recent study involving over 520,000 participants and more than 104,000 events, it was found that circulating linoleic acid and arachidonic acid were causally associated with a decreased risk and increased risk of coronary heart disease, respectively (165). In another recent study involving over 1 million participants and 137,000 events, it was found that circulating arachidonic acid was causally associated with an increased risk of both coronary heart disease and ischemic stroke, and that this effect was directly proportional to the absolute increase in apo-B [Figure 37] (166). It is important to recognize that when consumed in the background of a Western-type diet, circulating arachidonic acid does not reflect the intake of linoleic acid, the predominant omega-6 fatty acid commonly derived from grains, legumes, nuts, and seeds, but rather direct intake of arachidonic acid, the major sources being red meat, poultry, fish, eggs, and dairy (167 168).
Importantly, in this study genetically predicted circulating arachidonic acid associated with higher levels of apo-B, LDL, apo-A-I, and HDL, and lower levels of triglycerides, which is the expected effect of substituting carbohydrate with saturated fat, and not arachidonic acid intake (35 166). This is compatible with the evidence that in Western populations, circulating arachidonic acid is a surrogate marker of saturated animal fat intake, which is most commonly displaced by refined sources of carbohydrate (4 167). Taken together, this indicates that in the influence of a Western-type diet, genetic variants associated with higher levels of circulating arachidonic acid may actually increase levels of these fatty acids by causing a preference for a diet richer in saturated animal fat, typically at the expense of a lower intake of refined sources of carbohydrate. This study also provides further strong causal evidence that apo-B, representing the total number of atherogenic particles, is the primary lipid determinant that explains the relationship between the lipid-modifying effect of diet and atherosclerotic cardiovascular disease, with little or no benefit of modifying the size of lipoprotein particles, or raising HDL, independent of apo-B [Figure 37, Table 1].
Epidemiological evidence of a direct benefit of a cholesterol-lowering diet is indicated in part by the findings from recent meta-analyses of prospective cohort studies of an adverse effect of saturated fat intake on the risk of cardiovascular disease, particularly for fatal events and among diabetics, and despite being compared to predominantly refined sources of polyunsaturated fat and carbohydrate [Figures 4, 38] (169 170 171 172). It is important to recognize that both clinical trials and prospective cohort studies examining saturated fat intake inherited a number of important problems that may have limited the power to detect a benefit of reduced intake. For clinical trials, these may include short duration of follow-up, limited changes to intake, and increases in industrial trans-fat intake, and prospective observational studies, substitution with predominantly refined sources of carbohydrate, overadjustment for intermediate risk factors, measurement error, residual confounding, and reverse causality (4 164 169 170 171 172 173).
The inconsistencies between studies examining saturated fat intake may also in part reflect selection bias. If participants consuming a high-saturated fat diet die of another cause before experiencing a major cardiovascular event, this may result in competing risk bias and obscure the relationship with cardiovascular disease. Similarly, if participants consuming a high-saturated fat diet who are at risk of cardiovascular disease are excluded either due to death prior to study baseline or a disqualifying pre-existing condition, as may be expected in studies recruiting older participants, this may result in the survivorship bias in which only the healthiest participants consuming a high-saturated fat diet are selected for inclusion. These selection biases were the focus of a recent study examining the effect of lipid-modifiers on ischemic stroke (65). Even though clinical trials have established that lowering apo-B reduces the risk of both total and ischemic stroke, higher cholesterol levels and saturated fat intake are often associated with a reduced risk in observational studies (34). This paradox has been explained largely by the fact that death from coronary heart disease typically occurs at younger ages than from stroke (65).
As a result, a study of the association of lipid modifiers with stroke among the living will automatically select on surviving high lipids and on surviving competing risk of prior death from IHD [ischemic heart disease] due to shared etiology between IHD and stroke. Some people dying from genetically high lipids and others dying from IHD before recruitment into a stroke study will leave a shortage of people available to recruit with genetically high lipids and susceptibility to stroke, thereby obscuring any effect of lipids or lipid modifiers on stroke.
To illustrate this phenomenon, the authors demonstrated using Mendelian randomization that statin therapy associated with a trend towards an increased risk of ischemic stroke in an unadjusted model, but with a significantly reduced risk after multivariable adjustment for major causes of survival and stroke (65). Notably, however, benefit was observed only when the analysis was restricted to the one available study that recruited younger participants of an age before cardiovascular disease typically occurs (65). While Mendelian randomization occurs at conception, survival to recruitment will mean that these studies are still susceptible to survivorship bias, particularly if older participants are recruited (65). Although the findings from this particular study cannot necessarily be generalized to non-Western populations, particularly Asian populations that are at higher risk of different stroke subtypes, a recent large Mendelian randomization study in Chinese adults found evidence that lower LDL-C, even down to low levels significantly reduces the risk of both total stroke and total cardiovascular events (174). Associations between an exposure and a higher risk of late-onset disease, including the case of lipid-lowering therapies and risk of ischemic stroke, can often be an indicator of benefit on lifespan, and should not necessarily be confused as evidence of harm.
While lowering the concentration of apo-B is crucial for reducing the risk of cardiovascular disease, it is important to recognize that clinical trials of cholesterol-lowering therapies have found that some people still experience plaque progression and major cardiovascular events even when achieving very low levels (32). This indicates the importance of other therapies, including a plant-based diet, that have benefits that extend beyond lipid-lowering in order to eliminate residual risk. Indeed, a substantial body of clinical evidence from both human and primate studies strongly supports the notion that substituting animal foods with high-quality plant foods reduces the risk of cardiovascular disease and promotes the regression of atherosclerosis, both independent of and in addition to changes in blood lipids [Figures 16-17, 20, 35, Table 4]. This notion is further supported by the findings from 100 separate prospective cohort studies indicating that modest increments in intake of dietary fiber and antioxidant phytochemicals are associated with about a 25% reduced risk of cardiovascular disease, a reduction greater than that explained by changes to traditional risk factors alone (44 175 176). As the intake of these nutrients often act as a surrogate marker of high-quality plant food intake, the benefits observed in these studies do not likely reflect the effect of a single specific nutrient, but rather, a combination of very numerous phytonutrients. There are thousands of known phytochemicals, and the complex interaction between these very numerous chemicals likely importantly contribute to the health benefits of minimally refined plant foods (177). The benefits of a plant-based diet are not likely fully appreciated by the common oversimplistic reductionist approach of examining the effect of singular isolated nutrients, which only at best help determine the absolute minimum likely benefit. The whole is greater than the sum of the parts.
The preponderance of evidence from over a century of atherosclerosis research has established that a minimally processed plant-based diet rich in fiber and antioxidant phytochemicals is the most optimal diet for reducing the risk of cardiovascular disease and promoting the regression of atherosclerosis. The benefits described in the studies published by Dr. Ornish and Dr. Esselstyn are by no means outliers, but rather supplement a substantial body of existing evidence from the scientific literature, including; over 1,000 controlled dietary experiments evaluating the effect of animal and plant-based nutrients on cardiovascular risk factors; over 200 clinical and genetic studies evaluating the effect of apo-B-containing lipoproteins on the risk of cardiovascular disease; 100 prospective cohort studies evaluating the effect of plant-based nutrients on the risk of cardiovascular disease; and thousands of experiments in over 100 species and breeds of animals, including more than 20 species of nonhuman primates evaluating the effect of animal and plant-based nutrients on the progression and regression of atherosclerosis [Figures 5, 7-18, 20-24, 30-35, 38-41, Tables 3-5]. While the available evidence provides support for the classical diet-heart hypothesis, it also indicates room for meaningful improvement through a greater focus on minimally refined plant-based diets, which reduce risk both independent of and in addition to changes in blood lipids. The common arguments of the critics of a lack of substantial evidence that animal foods promote atherosclerosis, or that this evidence is primarily limited to laboratory experiments in obligate herbivores with vast genetic differences to humans demonstrates an ignorance of a century of research on the subject.
The Grass Pipe Dream
The critics commonly argue that populations that traditionally subsisted on diets almost exclusively based on foods derived from animals consuming a natural, or “grass-fed” diet have very low rates of chronic and degenerative diseases that are common to the industrialized world. They argue that, therefore, much of the evidence of adverse effects of animal foods from studies carried out in the industrialized world cannot be extrapolated to foods derived from animals consuming a natural diet, particularly in the context of a low-carbohydrate, unprocessed diet. A particular interest in imitating the highly carnivorous diets of certain indigenous populations can be traced back to the works of Vilhjalmur Stefansson, an Arctic explorer who lived together with the Canadian Inuit between 1906-1907. Stefansson argued, despite a lack of extensive clinical evidence, that the Inuit were largely free of what he referred to as "diseases of civilization" while adhering to their pre-modern lifestyle and diet. What is often not appreciated is that Stefansson also emphasized the low rates of chronic diseases observed in traditional living populations that subsisted on plant-based diets, such as the Hunza of Kashmir (178). While a large body of evidence published over the following century consistently demonstrated low rates of many chronic and degenerative diseases among predominantly plant-based populations, the evidence has also demonstrated a significant burden, particularly of atherosclerotic and chronic inflammatory diseases among traditional living populations subsisting on diets rich in naturally derived animal foods.
Evidence based on careful clinical examinations has consistently shown a high prevalence of atherosclerosis among the Inuit, including pre-European contact populations predating the observations of Stefansson by up to 1,500 years, and as early as in the second decade of life [Table 6, Figure 39] (179 180 181 182 183 184 185). Literature reviews have also found that the claims of low incidence of atherosclerotic disease are based on scant clinical evidence (182). Arne Høygaard, a Norwegian physician, studied an Inuit population in Southeast Greenland during the 1930s that maintained a traditional lifestyle of hunting and fishing, and adhered to a traditional diet estimated to include only 3% of energy from carbohydrate (183). Høygaard noted based on clinical examinations, including X-ray evidence, that among Inuit described as "living mainly on primitive diet”:
Arteriosclerosis was frequently found even in persons below 40.
Consistent with these observations, in 1940, based on decades of clinical practice and reviewing reports of medical officers dating back more than 180 years, Aage Bertelsen, often considered the father of Greenland epidemiology, stated in regards to the mortality patterns among the Greenland Inuit (182):
...arteriosclerosis and degeneration of the myocardium are quite common conditions among the Inuit, in particular considering the low mean age of the population.
 |
Figure 39. Evidence of coronary atherosclerosis observed in a pre-European contact Alaskan Inuit dating back 1,600 years ago. From Zimmerman, 1993 |
Høygaard also observed a high prevalence of osteoporosis, rheumatoid arthritis, among other skeletal and chronic inflammatory diseases among the hunter-gatherer Inuit (183). These findings are consistent with the high prevalence of these disorders observed in multiple other post- and pre-European contact Inuit populations (181 186 187). Moreover, a recent study found that saturated fat intake predicted poor bone strength among the Inuit, indicating that a high intake of animal foods can likely partly explain observations of a high prevalence of osteoporosis (188). It is important to recognize that while the Inuit have been argued as a population that has traditionally adhered to a ketosis-inducing diet, it is a common misconception that the Inuit frequently exhibit ketosis. Both Høygaard and multiple other researchers have consistently found that the traditional living Inuit are very resistant from entering ketosis, with ketones typically only detected during starvation, and even then, only in small amounts (113 183). Taken together, the evidence strongly refutes both the arguments that the Inuit are an example of a population that sustain prolonged periods in ketosis, and that an otherwise ketosis-inducing unprocessed diet protected the Inuit from chronic diseases.
The critics also commonly argue that the Maasai (or Masai), a semi-nomadic ethnic group that inhabits Kenya and Tanzania have very low rates of chronic diseases, including atherosclerotic cardiovascular disease. The interest in the diet and health of the Maasai can be traced back to the findings of the field research carried out in the 1920s and published by John Boyd Orr, who portrayed the Maasai as subsisting chiefly on large amounts of meat, milk, and blood. Orr, a spokesperson for British Milk Marketing, a self-proclaimed “champion of the British dairy industry”, and outspoken against the rising trend in plant-based diets in Britain at the time, has been criticized for omitting relevant data from reports of comparisons of diet and health between the Maasai and other African ethnic groups (189). Interestingly, Orr did however recognize the findings that 80% of the Maasai studied reported rheumatoid arthritis and habitual constipation (189).
Orr has in particular been criticized for neglecting how dietary patterns earlier in the life of the ethnic groups studied may have influenced the observations on health. The dietary patterns of the Maasai throughout history have by no means been constant, in part due to prolonged periods of significant loss of livestock, observed dating back to at least the 1890s when the Maasai were temporarily forced to subsist on a plant-based diet (189). Although examined in later decades, systematically documented dietary surveys and observations of energy expenditure have consistently demonstrated that the earlier claims of such high intakes of animal foods were likely exaggerated (190 191). Perhaps most relevant to the later studies examining the health of the Maasai, was that beginning around 1960, the Maasailands experienced severe drought and flooding, and with the addition to the then-recent regulations imposed on grazing range, resulted in the loss of two-thirds of livestock (192).
Two autopsy studies have examined the prevalence of atherosclerosis in the Maasai, almost all of who had died during the 1960s. The larger study, carried out by George Mann and colleagues, and supported by the National Live Stock and Meat Board, found definitive and extensive atherosclerosis among 50 Maasai men who died between 1966 and 1970 [Figure 40]. The authors of this study questioned the validity of the smaller autopsy study in only 10 men that suggested an absence of atherosclerosis, due to questionable methodology, and the failure to provide even basic information about the selection of subjects who were presumed to be Maasai (193). Mann, a prominent cholesterol skeptic, however, argued that the extensive atherosclerosis observed in the Maasai may be explained by the consumption of highly processed foods that were available to the Maasai in small quantities. This explanation is questionable, however, given that atherosclerosis was actually observed to be significantly more extensive among elderly Maasai compared to U.S. men of a similar age, despite evidently, on average, consuming lower intakes of processed foods while also evidently having a lower rate of other unfavorable risk factors, including physical inactivity, smoking, obesity, and high blood pressure [Figure 40].
Mann also downplayed a possible adverse effect of animal foods by emphasizing the observed trend of less extensive atherosclerosis in those who died during the third decade of life following the period, typically between the ages of 12 and 30, that Maasai men spend as Moran warriors where they are portrayed to subsist on a diet purely of milk, meat, and blood. Importantly, this is also the period when they are the most active. However, given both the loss of the great majority of livestock in the Maasailands during the 1960s, and tribal restrictions in place on game meat, the Moran would have, leading up to study, subsisted on either a predominantly plant-based diet, or a diet that was severely energy restricted. Thus, any actual reduction in severity of atherosclerosis may simply be explained by the evidence established from randomized controlled trials that energy restriction combined with exercise improves markers of atherosclerosis (194 195).
 |
| Figure 40. Coronary artery atherosclerosis measured by intimal thickness in Maasai men compared to U.S men. From Mann et al., 1972 |
While some studies have reported low cholesterol levels in the Maasai, there are several important factors, in addition to the exaggerated quantification of animal food intake that can help explain this apparent paradox, and has been reviewed in detail elsewhere. Nevertheless, high cholesterol levels have been observed in some nomadic-living Maasai-speaking populations (196). Moreover, a recent study found that a high intake of red meat was associated with a more than 50% increased risk of high cholesterol in the Maasai, indicating that the traditional diet rich in grass-fed animal foods can help explain the high prevalence of atherosclerosis observed in the Maasai (197).
The traditional living Inuit and Maasai have both been observed to have very physical lifestyles while subsisting on relatively low-calorie diets, often sustaining periods of starvation. While these lifestyles factors may help explain low rates of certain disorders, these populations have nevertheless been observed to have a high prevalence of atherosclerotic and chronic inflammatory diseases. This raises considerable concerns as to how the observations of these ethnic groups have been extrapolated to argue that people living in the industrialized world with comparatively sedentary lifestyles and unlimited access to food would somehow benefit from consuming unlimited quantities of fatty animal foods. More relevant, may be the findings of the health of traditional living populations that subsisted on a more caloric-rich animal-based diet while adhering to an only moderately active lifestyle. Perhaps of particular relevance are the findings on the health of the nomadic pastoralist populations of Central Asia, who have been observed to subsist on diets almost exclusively based on large quantities of meat, fat, and milk from grass-fed animals, while often actively avoiding agriculture produce, including grains [Table 7] (198).
In good legendary style, the pure Central Asian nomads eat only meat, marrow, and milk products {preferably ferments}. They despise farmers, farming, and grain…
Almost 2,500 years ago, Hippocrates described the high prevalence of cardiovascular risk factors, including obesity, male impotency, and female sexual dysfunction among the nomadic Scythian of the Eurasian Steppe, described to subsist chiefly on boiled meat, milk, and cheese (199). Similarly, in the 13th century, cardiovascular risk factors, including obesity and gout were commonly observed among the Mongol of the Golden Horde who subsisted on a largely similar diet, with definitive evidence of atherosclerosis observed in mummified remains of the nomadic Mongol dating back to the 15th century [Table 7, Figure 41] (200 201). Gout, a disease that has been described since ancient times, and commonly observed among nomadic pastoralist populations, was recently associated with the intake of both meat and seafood in a meta-analysis of 19 studies [Table 7] (202). Gout has even commonly been observed in the fossils of omnivorous and carnivorous dinosaurs, including the Tyrannosaurus Rex, consistent with the evidence that reptiles are also susceptible to dietary, particularly meat-induced increases in uric acid levels (203 204). As with other chronic and degenerative diseases, this indicates that the intake of flesh, independent of quality is a cause of gout.
 |
| Table 7. Evidence of the prevalence of cardiovascular disease and cardiovascular risk factors among nomadic pastoralist populations of Central Asia |
 |
Figure 41. |
In 1925, Maxime Hans Kuczynski, a German-Peruvian physician reported on the nomadic pastoralists, evidently of mixed-descent, from the steppe of Central Asia and Northwest China. Kuczynski observed that these pastoralists subsisted almost exclusively on very large quantities of meat and milk from grass-fed animals, and observed a high prevalence of not only obesity, gout, and male impotency noted in earlier observations of pastoralist populations, but also extensive premature atherosclerosis, nervous system disorders, and habitual constipation (205 206). Kuczynski, referencing the then-recent breakthrough research by Anichkov, attributed the high rates of cardiovascular disease to a cholesterol-rich diet (206).
They get arteriosclerosis in an intense degree and often at an early age as shown by cardiac symptoms, nervous disorders, typical changes of the peripheral vessels, nephrosclerosis and, finally, apoplectic attacks. Even in men thirty-two years old I frequently observed arcus senilis.
Kuczynski also examined a neighboring population of Russian peasants, observing that they did not experience these disorders while subsisting on a predominantly plant-based diet based on soup, bread, pickles, and potatoes, but with large quantities of alcohol (205). Relatedly, in another publication from the same year, only one pronounced case of atherosclerosis was observed in a study of 100 autopsies from Shanghai, where the population also subsisted on a predominantly plant-based diet (113). Moreover, in the 1960s, it was observed that the prevalence of coronary heart disease among the nomadic pastoralists from Xinjiang in Northwest China who consumed large quantities of animal fat from grass-fed animals, was eight times higher than that of other populations within Xinjiang, and up to more than 30 times higher than populations in other parts of China which subsisted on diets very low in animal fat (207).
By the mid-1930s, decades before the globalization of highly processed diets, multiple researchers had already reported on the correlation between diets rich in animal foods and the global geographic distribution of atherosclerotic cardiovascular disease. In 1934, S. R. Rosenthal reviewed 28 studies from across Africa, Asia, Europe, and North and South America, and observed in addition to a correlation between fatty animal foods and the geographical distribution of atherosclerosis, a significant increase in incidence among populations transitioning to a diet rich in animal foods, as well as a significant decline in incidence among populations temporarily forced to subsist on a plant-based diet (208):
In no race for which a high cholesterol intake (in the form of eggs, butter and milk) and fat intake are recorded is atherosclerosis absent.
Rosenthal also observed a very low prevalence of atherosclerosis among populations consuming plant-based diets, with intakes of carbohydrate as high as 91% of total energy (208). Numerous studies from the following decades confirmed the findings of a low prevalence of atherosclerosis among populations consuming almost exclusively plant-based diets, including populations with even more extreme intakes of carbohydrate. This importantly includes evidence from autopsy studies of a very low prevalence of atherosclerosis among the Okinawans and the Papua New Guinea highland populations, observed to consume on average up to 860 and 540 gm/day of carbohydrate, respectively [Tables 8-9] (113 209 210 211 212 213 214). The findings from Papua New Guinea, particularly from the highland regions are striking given the very high exposure to hazardous smoke due to both a high prevalence of tobacco smoking and a lifestyle of spending up to 12 hours a day in smoke-filled homes as a result of common heating practices (209). On the other hand, the findings from the autopsy studies in the Okinawans are striking given the low prevalence of atherosclerosis observed even among the very elderly. These latter observations are consistent with the autopsy findings by Kuczynski, who noted a large absence of atherosclerosis among extremely elderly Russians consuming predominantly plant-based diets (205). These observations are also consistent with the suggestive evidence of an absence of heart disease among the elderly Hunza over the age of 90 (215).
The notion that imitating the highly carnivorous diets of certain indigenous populations can promote health is simply a pipe dream. Arguments that naturally derived animal foods are inherently healthy plays on the appeal to nature fallacy, and lacks as much credibility as the argument that organic tobacco is healthy. Evidence from around the globe has consistently demonstrated a high prevalence of multiple chronic and degenerative diseases, often by the fourth decade of life among traditional living populations subsisting on diets very rich in, or almost exclusively based on foods derived from animals consuming a natural diet [Figures 39-41, Tables 6-9]. These conditions commonly include atherosclerotic cardiovascular disease, chronic inflammatory disorders, constipation, erectile dysfunction, and when energy intake is high, obesity. As such, population studies provide further evidence that the adverse effects of animal foods are consistent even in the context of a low-carbohydrate, unprocessed diet, rich in naturally derived animal foods. Interestingly, a high prevalence of conditions, including atherosclerotic and chronic inflammatory diseases has commonly been observed in these populations even in the absence of a number of traditional risk factors, including high cholesterol, high blood pressure, obesity, and physical inactivity. This is consistent with other lines of evidence indicating that the adverse effects of substituting high-quality plant foods with animal foods are both independent of and in addition to changes to traditional risk factors [Figures 35, Table 4]. While the totality of evidence strongly refutes the notion that a diet rich in naturally derived, or “grass-fed” animal foods can be health-promoting, some of Stefansson’s hypotheses have nevertheless stood the test of time. These include the beneficial effects of an active lifestyle and traditional diets predominated by unrefined plant foods, which may importantly explain the prolonged delay in decline in cardiovascular health observed in the elderly of a number of long-lived populations.
Penile-Artery-Clogging-Saturated-Fat
When fed diets rich in cholesterol and saturated fat for several years, primates develop atherosclerotic plaque in the pelvic and penile arteries, causing erectile dysfunction (ED) [Tables 10-11] (147). In contrast, the substitution of animal protein with plant protein, particularly soy protein prevents the progression of atherosclerotic plaque formation in the pelvic (iliac) arteries of nonhuman primates without adversely affecting either testosterone levels or testicular development [Figure 42] (159 162 163). Experiments in swine, rats, mice, and rabbits have also found that impaired blood flow resulting from atherosclerosis and endothelial dysfunction induced by a diet rich in cholesterol and saturated fat causes ED (147 216 217 218). Thus, evidence from animal models strongly indicates that ED and atherosclerotic cardiovascular disease share a similar etiology. There is a substantial body of supporting evidence that humans are not an exception to these animal models of ED. A recent meta-analysis of 41 clinical trials found that neither soy nor isoflavone intake affects male reproductive hormones (219). Evidence from over 1,000 controlled dietary experiments has established that substituting high-quality plant foods with animal foods, particularly those rich in saturated fat, increases the concentration of apolipoprotein B (apo-B)-containing lipoproteins and, in turn, evidence from over 200 clinical and genetic studies has established that apo-B causes atherosclerotic cardiovascular disease [Figures 5, 7-15, 21-24, 30-31, Table 1]. Moreover, direct evidence from clinical and genetic studies strongly indicates that major sources of saturated fat increase the risk of cardiovascular disease proportional to both the increase in apo-B and duration of exposure to higher intake [Figures 21-22, 36-37].
Multiple lines of evidence provide support for the notion that apo-B is an important determinant of ED in humans, and that this effect is also mediated by endothelial dysfunction and atherosclerotic plaque formation. A recent meta-analysis of randomized controlled trials found that statins produce a clinically relevant improvement in erectile function, despite deleterious off-target effects of lowering testosterone (220). Epidemiological and genetic studies have also found suggestive evidence that LDL-C and apo-B increase the risk of ED (221 222 223). In addition, epidemiological evidence indicates that a higher concentration of apo-B also reduces the efficacy of sildenafil (Viagra) (224). Moreover, a recent meta-analysis of epidemiological studies found that both measures of atherosclerosis and endothelial dysfunction, including flow-mediated dilation (FMD), were strong predictors of ED, risk factors found to be causally associated with LDL in clinical and genetic studies (109 225 226 227 228 229).
Evidence from a meta-analysis of randomized controlled trials indicates that compared to low-fat diets favoring low-quality sources of carbohydrate, low-carbohydrate diets favoring animal protein and fat reduce FMD by about 1% [Figure 43] (230). This is unlikely a trivial decrease, given that observationally, each 1% decrease in FMD is associated with a 15% increased risk of cardiovascular disease (231). Importantly, this is a magnitude of reduction similar to that observed for cigarette smoking, and likely a considerable cause for concern given the evidence that endothelial dysfunction is an important determinant of the effect of smoking on ED (232 233). Evidence of an adverse effect of low-carbohydrate diets on endothelial function is also supported by numerous additional intervention studies, and has generally been observed to be most pronounced when the diets were rich in saturated fat and when compared to diets rich in unrefined sources of carbohydrates (234 235 236 237). In one particular intervention study which found that an Atkins diet impaired FMD compared to an Ornish diet, saturated fat was shown to be independently associated with a very substantial reduction in FMD [Figure 44] (234). These findings are consistent with a substantial body of evidence from intervention studies in both humans and nonhuman primates that diets rich in cholesterol and saturated fat cause endothelial dysfunction (238 239 240 241 242 243 244 245).
 |
| Figure 43. Effect of low-carbohydrate diets on flow-mediated dilatation (%) compared to low-fat diets of non-specific quality in a meta-analysis of 7 randomized controlled trials |
The Game Changers documented two small experiments indicating that substituting organic, naturally derived animal foods with plant-based substitutes significantly improved both endothelial function and erectile function. These findings are compatible with the observations of a high prevalence of male impotency among nomadic pastoralist populations subsisting chiefly on foods derived from grass-fed animals. Almost 2,500 years ago, Hippocrates described the high prevalence of impotency among the nomadic Scythian of the Eurasian Steppe, although as originally hypothesized, this may have been partly attributed to excessive horse-riding (199 206). A century ago, Kuczynski observed a high prevalence of impotency among the Central Asian nomadic pastoralists, and attributed this directly to their “pure meat-milk-diet” (205 206). Kuczynski observed that in contrast, the neighboring peasant population generally retained sexual functionality into old age, attributing this primarily to a plant-based diet (205).
Repeatedly I found at the age of about seventy years no signs of arteriosclerosis, no arcus senilis, etc.; they were men of youthful appearance, with no grey in their still abundant growth of hair, and with their sexual functions still intact.
While the high rates of ED observed in these pastoralist populations may partly reflect a high prevalence of obesity, nomadic pastoralists of Kenya with a low energy intake and low body fat have also been observed to experience an age-related increase in prevalence of erectile dysfunction at least as great as that observed in industrialized nations with a high prevalence of obesity and other ED risk factors (246 247).
Most recently, several studies observed that the healthful plant-based diet index (hPDI), which evaluates the effect of diets richer in high-quality plant foods, including whole grains, fruits, vegetables, nuts, legumes, tea and coffee, and in some studies, vegetable oils, was associated with a substantially reduced risk of erectile dysfunction in both Asian and Western populations [Figure 45] (248 249). These findings indicate that a 10-unit reduction in the hPDI, representing a reduction of up to about 3 servings of high-quality plant foods per day increases the risk of ED by a magnitude comparable to that observed for smoking one pack of cigarettes per day (250). These findings are also compatible with other clinical and epidemiological evidence indicating that high-quality plant food intake and a high ratio of unsaturated to saturated fat intake reduces the risk of ED (251 252 253). Evidence indicates that the benefit of a plant-based diet for preventing ED also extends beyond lipid-lowering. Notably, a recent Mendelian randomization study found causal evidence that improvements to both systolic blood pressure and insulin resistance, risk factors commonly improved by plant-based diets, also likely reduce the risk of ED (221). Taken together with a recent randomized controlled trial coauthored by Caldwell Esselstyn indicating that a plant-based diet can reverse vascular dysfunction, these findings may help explain the striking reports of ED resolving in a number of Esselstyn’s patients adhering to a whole-foods plant-based diet (254 255).
 |
| Figure 45. Association between a high or 10-unit increase in healthful plant-based dietary index (hPDI) and erectile dysfunction in a meta-analysis of cross-sectional studies. * Awaiting peer review |
Evidence indicates that diets rich in animal foods may also adversely affect other measures of male fertility. Consistent with the findings of the adverse effects on mating success in nonhuman primates, a recent meta-analysis with 285 estimates from 52 studies across multiple strains of mice, rats, and rabbits found that a high-fat diet, typically rich in cholesterol and saturated fat adversely affected more than a dozen measurements of male reproductive success, including sperm concentration and quality (256). Similarly, human studies have found that major sources of saturated animal fat are observationally associated with both a lower total sperm count and concentration, even in nations where bovine growth hormone use in cattle is banned (257 258 259 260 261). Moreover, a recent Cochrane review of randomized controlled trials found suggestive evidence that antioxidants can improve male subfertility, consistent with other clinical and epidemiological evidence indicating that high-quality plant food intake improves sperm quality (262 263 264 265).
A substantial body of evidence from clinical, genetic, epidemiological, and animal studies strongly indicates that compared to a high-quality plant-based diet, a diet rich in animal protein and saturated fat, regardless of whether in the context of a low-carbohydrate, unprocessed diet, rich in naturally derived animal foods, causes atherosclerosis and endothelial dysfunction and, in turn, impairs the regulation of penile blood flow and increases the risk of erectile dysfunction [Figures 5, 7-45, Tables 1-11]. Suggestive evidence also indicates that the adverse effects of a diet rich in animal foods extend to other measures of male fertility, including sperm quality. In contrast, evidence indicates that a high-quality plant-based diet is optimal for male subfertility, and that the major beneficial effects, including the reversal of atherosclerosis, vascular dysfunction, type II diabetes, and hypertension may not only reduce the risk of developing erectile dysfunction and improve the efficacy of pharmaceutical treatments, but even help to reverse moderate to severe cases of atherosclerotic erectile dysfunction [Figures 29-31, 33-34, 42-45, Table 5].
Growing the Wrong Cells
In 1908, W. Roger Williams reported on the correlation between diets rich in animal foods and the global geographic distribution of cancer (266). Similar to the early observations on atherosclerosis, Williams noted significant increases in cancer incidence among populations transitioning to diets richer in animal foods, and a very low incidence among populations subsisting almost exclusively on plant-based diets. Williams also similarly noted elevated rates of cancer among carnivorous compared to herbivorous animals. Williams concluded based on a substantial number of observations and statistics collected from even the more remote parts of the globe, that there was convincing evidence that diets rich in animal protein are a cause of cancer. Earlier, in 1898, Williams elaborated on this in the Lancet (267):
Probably no single factor is more potent in determining the outbreak of cancer in the predisposed, than excessive feeding… Many indications point to the gluttonous consumption of proteids especially meat which is such a characteristic feature of the age, as likely to be specially harmful in this respect… When excessive quantities of such highly stimulating forms of nutriment are ingested by persons whose cellular metabolism is defective it seems probable that there may thus be excited in those parts of the body where vital processes are still active such excessive and disorderly cellular proliferation as may eventuate in cancer. No doubt other factors co-operate besides those I have already mentioned, and among these I should be inclined to name deficient exercise, and probably also lack of sufficient fresh vegetable food.
Williams' observations of cancer in animals were recently confirmed by an analysis of over 110,000 adult zoo mammals, which found that carnivorous mammals experienced the highest age-controlled cancer-related mortality rates (268). What is perhaps even more remarkable, was that even based on the evidence available over 120 years ago, Williams was able to hypothesize that “cellular proliferation” in response to a diet rich in animal protein is an important determinant of cancer. This was more than a century before Dr. Colin Campbell highlighted similar concerns in the bestseller book, The China Study. This hypothesis of Williams, and Campbell who followed is highly compatible with the rapidly growing body of evidence from recent decades implicating that insulin-like growth factor 1 (IGF-1), which stimulates the proliferation of cells, is both modifiable by dietary protein and a cause of cancer [Figures 46-47]. Most recently, large Mendelian randomization studies have found that IGF-1 is causally associated with an increased risk of multiple major cancers (269). Separately, a growing body of evidence from clinical and epidemiological studies strongly indicates that the intake of protein, particularly from dairy and other animal sources increases IGF-1 (270 271 272 273 274 275 276) [Figure 46]. This notably includes the observations that compared to omnivores, vegans have significantly lower concentrations of IGF-1, with intermediate levels in lacto-ovo vegetarians (273 274).
While Mendelian randomization studies have often found a causal association between IGF-1 and an increased risk of certain cancers, the findings have not always been entirely consistent. Therefore, for this review, fixed-effects meta-analyses were carried out to examine the causal association between IGF-1 and site-specific cancer. In meta-analyses including over 241,000 cases of cancer, higher IGF-1 levels were causally associated with an increased risk of breast, prostate, and colorectal cancer [Figure 47] (269). These findings are remarkably consistent with the evidence from large meta-analyses of observational studies, strongly indicating that IGF-1 is an independent cause of these cancers (275 277 278 279). Moreover, Mendelian randomization studies have also found suggestive evidence that atherogenic lipids are causally associated with an increased risk of breast, prostate, and colorectal cancer, further implicating that animal food intake is a cause of these cancers [Figure 12] (280 281 282 283).
 |
| Figure 46. Effect of dairy intake on Insulin-like growth factor 1 (IGF-1) from a meta-analysis of 28 studies involving 27,408 participants. From Harrison al., 2017 |
There is also a substantial body of direct evidence implicating that the intake of animal foods relative to high-quality plant foods increases the risk of breast, prostate, and colorectal cancer. For colorectal cancer incidence, meta-analyses of prospective cohort studies have found that the intake of red meat and dietary heme iron is associated with an increased risk, and fiber-rich plant foods are associated with a decreased risk (24 30 284 285). Randomized controlled trials have also found that low carbohydrate diets and the intake of meat and heme iron adversely affect, while fiber-rich plant foods improve risk factors for colorectal cancer (24 30 286 287). The effect of dietary fat on colorectal cancer has been one of the most studied relationships between diet and chronic disease examined using Mendelian randomization (288 289 290). However, the findings have not always been entirely consistent. Therefore, for this review, fixed-effect meta-analyses were carried out to examine the causal association between circulating essential omega-3 and omega-6 fatty acids and colorectal cancer. In meta-analyses involving over 34,000 cases of colorectal cancer, circulating short chain and long-chain polyunsaturated fatty acids, which are surrogate markers of plant and animal fat intake, were associated with a decreased and increased risk of colorectal cancer, respectively [Figure 48]. These findings indicate that the intake of fat derived not only from terrestrial animals, but also from marine animals may increase the risk of colorectal cancer.
For breast cancer incidence, meta-analyses of prospective cohort studies have found that the intake of red meat, eggs, and saturated fat is associated with an increased risk, and fiber and antioxidant-rich plant foods are associated with a decreased risk (291 292). In the Women’s Health Initiative, a randomized controlled trial involving almost 50,000 participants, it was found that death following breast cancer was statistically significantly lower during the 8-year intervention period in the experimental low-fat group, with a statistically significant 21% reduction in death from breast cancer that became apparent after almost 20 years of follow-up [Figure 2] (293). This was despite only small reductions in intake of animal foods and animal-based nutrients which were displaced largely by refined sources of carbohydrate (23).
For prostate cancer incidence, meta-analyses of prospective cohort studies have found that the intake of dairy is associated with an increased risk, and high-quality plant-based dietary patterns as well as antioxidant-rich plant foods, including soy, and certain fruits and vegetables are associated with a decreased risk (275 294 295 296 297). In the Prostate Cancer Lifestyle Trial led by Dean Ornish which examined 92 men with early-stage prostate cancer, it was found after 2 years of follow-up that only 5% of the experimental group that was advised to adopt a plant-based diet in addition to exercise and stress management had undergone conventional prostate cancer treatment, compared to 27% of the control group receiving usual care (298). Interestingly, IGF-1 levels actually increased in both groups, but more so in the experimental group which was associated with a 58-gm daily supplement of soy protein isolate (299). While the findings of an increase in IGF-1 is largely comparable to other trials examining the effects of soy protein, in this trial, IGFBP-1 levels, which potentially reduces the risk of cancer due to binding effects with IGF-1, was only increased in the experimental group and was associated with plant protein intake (299 300). Indeed, observational studies have found some, albeit weak, evidence that IGFBP-1 reduces the risk of prostate cancer (301). Interestingly, in a separate short-term placebo-controlled trial which examined 58 men at high risk of prostate cancer, while too small to necessary prove an effect, it was found that prostate cancer developed in only 6% of men who were provided with soy protein isolate, compared to 38% provided milk protein isolate (302).
While a substantial body of evidence indicates that a plant-based diet is optimal for reducing cancer risk, few epidemiological studies have actually examined the effects of substituting animal foods with high-quality plant foods on the risk of cancer. Most recently, the NIH-AARP study involving over 521,000 participants and more than 35,000 cancer deaths during 8.3 million person-years of follow-up found that substituting one whole egg per day with either a serving of nuts or legumes was associated with a 10% reduced risk of total cancer mortality (303). This was a greater benefit than observed for the substitution with any major source of animal protein. A recent pooled analysis of the Nurses’ Health Study (NHS I and II) and Health Professionals Follow-up Study (HPFS) found that substituting one serving per day of dairy with a serving of whole grains was also associated with about a 10% reduced risk of total cancer mortality [Figure 49] (304). A recent publication from the Adventist Health Study-2 also found that substituting median intakes of soy milk with dairy milk was associated with a 46% increased risk of breast cancer (305). Notably, in this study, the observed benefit of reduced dairy milk intake was most pronounced at very low intakes, a finding that few other studies have the power to detect due to a lack of participants with virtually zero intake. Similarly, an analysis from the NHS II found that substituting red meat with other sources of protein, including legumes during adolescence and early adulthood was associated with a reduced risk of breast cancer (306).
Recently, several large prospective cohort studies have examined the effect of substituting plant protein with animal protein on the risk of total cancer mortality (18 307 308 309). However, this evidence has not yet been reviewed in the form of a meta-analysis. Therefore, for this review, meta-analyses were carried out to examine the effect of substituting plant protein with specific sources of animal protein on the risk of total cancer mortality. In meta-analyses including up to 11 million person-years of follow-up, substituting plant protein with either red meat or egg protein was associated with a significantly increased risk of total cancer mortality [Figure 50]. The less clear association observed for other major sources of animal protein may in part be the result of competing risk of death from other causes, including a higher risk of cardiovascular disease [Figure 3]. Nevertheless, total white meat protein intake was found to be associated with an increased risk in women (RR=1.07 [1.01, 1.14] per 3% increase of energy) (307).
The discrepancy between the observed lack of an effect for substituting total plant protein with dairy protein, and the significant effect for substituting high-quality sources of plant protein with dairy on the risk of total cancer mortality observed from separate publications from the NHS and HPFS raises several important questions [Figures 3, 49] (18 304). While the association with an increased risk in the latter study may in part reflect a significantly larger number of participants or an adverse effect of dairy fat, the findings may be largely explained by the quality of plant protein substituted. As the intake of unprocessed sources of plant protein was almost universally low, plant protein intake would have been derived predominantly from lower quality foods, such as refined grains (3). Indeed, meta-analyses of prospective cohort studies have found that each modest increment in the intake of dietary fiber and antioxidant phytochemicals, which are surrogate markers of high-quality plant food intake, are associated with between about a 13% and 40% reduced risk of cancer incidence, indicating that the adverse effect of animal protein would be more pronounced when compared specifically to higher quality sources of plant protein (44 175).
A meta-analysis of 15 prospective cohort studies found that menthol cigarette smoking was associated with a 16% reduced risk of both lung and all cancers combined compared to nonmenthol cigarette smoking (2). However, when compared to never-smokers, menthol cigarette smokers were found to have a 103% increased risk of cancer incidence, demonstrating the critical importance of evaluating suitable comparison exposures for the study of cancer (2). Interestingly, this is actually the magnitude of increased risk indicated by prospective cohort studies for the substitution of approximately 15% of energy from plant protein of undefined quality with a combination of equal parts of energy from red meat and egg protein, assuming a linear dose-response relationship [Figure 50].
A substantial body of evidence from clinical, genetic, and epidemiological studies strongly indicates that substituting high-quality plant foods with animal foods can substantially increase the risk of cancer. In particular, recent causal evidence has found both that animal protein increases IGF-1 and that IGF-1 is a cause of cancer, indicating that cellular proliferation is an important determinant of the effect of animal protein on the risk of cancer, consistent with Williams' hypothesis from over 120 years ago. Taken together with recent evidence that growth hormone use has little or no benefit on either muscle strength or aerobic exercise capacity, the conventional advice to consume animal protein over plant protein may actually favor the growth of cancer tissue over that of lean muscle tissue (310). A shift of focus towards increasing exercise and consuming protein in moderation, primarily from high-quality sources of plant foods would likely provide a more health-promoting approach for modifying growth hormones. Considering that cardiovascular disease and cancer contribute to almost half of all global deaths, the evidence described here provides compelling support for the notion that compared to a high-quality plant-based diet, diets rich in animal foods contribute substantially to the global burden of premature death [Figures 1-50, Tables 1-11] (27).
Low-Carb High-Fatality
Evidence from thousands of studies spanning over a century of research has established beyond reasonable doubt that a high-quality plant-based diet rich in fiber and antioxidant phytochemicals is the most optimal diet for reducing the risk of major causes of death and disability in the general population [Figures 1-50, Tables 1-11]. However, as diet has a fairly uniform effect across multiple major causes of death, the study of diet will inevitably be complicated by competing risks (65 311). Competing risk of death from other causes can obscure or even reverse the true relationship with an outcome of interest, especially in the case of late-onset diseases (65). As most studies examining diet have limited power to control for competing risks, an analysis of the effect of diet on all-cause mortality will therefore often be more informative than for specific disease endpoints. Recent evidence examining the effects of lifelong cumulative exposure to disease risk factors and long-term substitution of animal foods with high-quality plant foods strongly indicates that the magnitude of benefit of long-term adherence to a high-quality plant-based diet is greater than may have previously been recognized. The totality of evidence indicates that comparatively, diets very rich in animal foods may increase the risk of premature death in excess of two-fold, a magnitude at least comparable to that observed for 50-pack years of cigarette smoking (70 311 312 313 314).
Evidence from over 100 clinical and genetic studies involving over 1.3 million participants has established that apolipoprotein B (apo-B) is the primary lipid determinant of atherosclerotic cardiovascular disease and total mortality [Table 1] (34 67 68 83 99). However, the therapeutic target of many commonly prescribed lipid-lowering therapies remains as LDL, often resulting in only modest reductions in apo-B. Even at the dosage used in large clinical trials, statin treatment reduces apo-B on average by about 30 mg/dl, a magnitude similar to that predicted by the substitution of only 3% of total energy from saturated fat with polyunsaturated fat (34 35). Thus, diet, in particular a high-quality plant-based diet that has benefits that extend beyond lipid-lowering, is evidently an important strategy for reducing apo-B and, in turn, the global burden of cardiovascular disease and premature death [Table 12].
Evidence from over 230 controlled dietary experiments has established that each 2% increment of energy from fatty animal foods can increase apo-B by up to about 10 mg/dl [Table 12] (28 29 30 35 37 40 315 316 317). In turn, a recent meta-regression analysis of randomized controlled trials involving 333,000 participants found that by 4.7 years of cumulative exposure, each 10 mg/dl increase in apo-B was causally associated with a 9% and 14% increased risk of total mortality and major adverse cardiovascular events, respectively [Figure 51] (34). Moreover, a recent Mendelian randomization study involving over 438,000 participants found that each 10 mg/dl increase in apo-B was causally associated with a 37% increased risk of major coronary events, indicating a 2.5-fold greater increase in risk compared to that observed for 5 years of cumulative exposure later in life [Figure 51] (33). This implies that a lifetime cumulative exposure to a 100 mg/dl higher apo-B may increase the odds of atherosclerotic cardiovascular disease by more than 20-fold. While such a magnitude of increased risk may be difficult to fathom, these findings are actually remarkably consistent with the observations that individuals who inherit homozygous familial hypercholesterolemia, resulting in an equivalent cumulative exposure to apo-B early in life, will commonly experience a major cardiac event in the first decade of life, and hence, are recommended cholesterol-lowering intervention by the age of 5 (81).
Evidence from clinical and genetic studies also strongly indicates that the risk of total mortality similarly increases with a greater duration of cumulative exposure to apo-B (70 98 99 318 319). A recent Mendelian randomization study found that a lifetime exposure to a 24 mg/dl higher apo-B was causally associated with a 43% increased risk of death before the 90th survival percentile, and a 164% increased risk after mutual adjustment for LDL-C and triglycerides (99). This indicates that each 10 mg/dl increase in apo-B increases the risk of premature death by between about 16% to 50% [Figure 51]. In another recent Mendelian randomization study it was found that a lifetime exposure to about a 1 mmol/l higher LDL-C was causally associated with a 32% increased risk of premature death after mutual adjustment for HDL and triglycerides (70). Importantly, this magnitude of increased risk was virtually identical to that observed for smoking initiation in the same study (70). These findings indicate a two-fold greater increase in risk of premature death compared to 5 years of cumulative exposure to apo-B-containing lipoproteins later in life (34 320). Taken together, the totality of evidence indicates that compared to a high-quality plant-based diet, a diet very rich in animal foods may increase apo-B by up to as much as 130 mg/dl, predicting a 200% increased risk of total mortality after 5 years, a risk that will continue to increase with a longer duration of adherence [Table 12, Figure 51].
The critics commonly argue citing observational evidence that a higher concentration of apo-B-containing lipoproteins, including LDL does not increase the risk of total mortality, and may actually decrease risk in the elderly. These arguments ignore a very substantial body of evidence from over 200 clinical and genetic studies involving 2 million participants and over 100,000 deaths that has unequivocally established that lowering apo-B and LDL-C reduces the risk of total mortality (34 66 69 70 74 98 99 320 321). Clinical and genetic studies indicate that this benefit on total mortality also extends to persons over the age of 75 (66 320 321). The discrepancies observed for mortality between some epidemiological studies can evidently be explained by the susceptibility of these studies to the survivorship bias and reverse causality (66). Reverse causality in this case is where a cholesterol-lowering comorbidity is both the cause of death and lower observed cholesterol levels. Reverse causality has also helped to explain the discrepancies in findings for blood lipids and Coronavirus disease 2019 (COVID-19). A recent Mendelian randomization study involving 1.3 million participants and more than 15,000 cases found that total cholesterol and apo-B are causally associated with an increased risk of developing COVID-19 (322). In contrast, a separate Mendelian randomization study involving 1.7 million participants and more than 36,000 cases found that COVID-19 infection lowers blood lipids, including LDL, thus explaining why some studies measuring cholesterol post-diagnosis have found lower levels among COVID-19 patients despite evidence of a causal positive relationship (323). The survivorship bias in this case is where the most susceptible participants with high cholesterol are excluded due to death prior to study baseline or a disqualifying pre-existing condition. The survivorship bias has also helped to explain a number of other paradoxes, including the observation that disadvantaged populations in the U.S. are observed to have a higher mortality rate than whites in every age group until about the age of 75, where it is then reversed (324). Similarly, this bias has been found to help explain the observation that among hospital-admitted survivors, smokers often have improved survival as smokers at high-risk are more likely to never reach the hospital (325).
Epidemiological studies predominated by individuals with familial hypercholesterolemia, who would generally maintain elevated cholesterol levels even in the presence of a cholesterol-lowering comorbidity, have also consistently found that higher cholesterol levels are associated with an increased risk of total mortality. In the largest study to date in patients with homozygous familial hypercholesterolemia, compared to on-treatment patients that achieved a total cholesterol level of <8.1 mmol/l, patients that achieved a total cholesterol of >15.1 mmol/l experienced a 1050% increased rate of total mortality over 25 years of follow-up (326). Similarly, in another study, compared to participants with an LDL-C of <4 mmol/l, participants with an LDL-C of >8.5 experienced a 717% increased rate of total mortality over 8.6 years of follow-up (327).
While the evidence strongly indicates that an animal-based diet can increase the risk of total mortality by up to more than three-fold predicted by changes to apo-B alone, a substantial body of evidence also indicates that the adverse effects extend beyond blood lipids, suggesting that the true risk is likely to be even greater [Figures 16-20, 28-29, 35, 39-41, 44-50, Tables 4, 6-9]. These include a higher body weight and blood pressure, risk factors that have been causally associated with a substantially increased risk of mortality in large Mendelian randomization studies (69 70). For example, it was found that a lifetime of cumulative exposure to each 1 mmHg increase in either diastolic or systolic blood pressure was causally associated with a 5% increased risk of death before the 90th survival percentile (70). Based on the changes to blood pressure observed in a recent controlled feeding trial, this indicates that a minimally processed animal-based ketogenic diet, even in the context of a very high intake of non-starchy vegetables (1 kg/day) and a moderately high ratio of unsaturated to saturated fat intake (>3:1), may increase the risk of premature death by up to a further 30% (50).
The critics commonly argue that the adverse effects of animal foods may be nullified when consumed in the context of a low carbohydrate, unprocessed diet, rich in naturally derived animal foods. A substantial body of evidence has strongly refuted these arguments. Numerous controlled dietary experiments have clearly demonstrated that the adverse effects of animal-based nutrients on disease risk factors, including apo-B, endothelial function, blood pressure, and body fat are consistent across extreme variations in macronutrient intake [Figures 8-9, 18-19, 43-44]. A high burden of dietary-related diseases, including cardiovascular disease, chronic inflammatory disorders, gout, constipation, and erectile dysfunction has also been commonly observed among traditional living populations consuming low-carbohydrate, unprocessed diets, predominated by naturally derived animal foods [Figures 39-41, Tables 6-7]. Additionally, experiments examining extreme variations in macronutrient intake have found that lower protein high-carbohydrate diets are associated with the longest lifespan in laboratory animals (328 329 330).
The evidence of the adverse effects of diets rich in animal foods in the context of a low-carbohydrate diet is also indicated by a recent meta-analysis of prospective cohort studies which found that animal-based and plant-based low-carbohydrate diets were associated with an increased and decreased risk of total mortality, respectively (331). However, as noted by the authors, a limitation of this meta-analysis was that the quality of carbohydrate was not considered, and as such, fat and protein would have displaced predominantly refined sources of carbohydrate. In contrast, a more recent publication based on National Health and Nutrition Examination Survey (NHANES) cohort evaluated low-carbohydrate diets in respect to the quality of carbohydrate intake, and found that each 20-percentile increase in the high-quality low-fat and plant-based low-carbohydrate scores was associated with an 11% and 9% decreased risk of total mortality, respectively (332). In addition, each 20-percentile increase in the animal-based low-carbohydrate score, in which both low and high-quality sources of carbohydrates were reduced in favor of an increased intake of animal protein and saturated fat, was associated with a 7% increased risk of total mortality (332).
For this review, an updated meta-analysis was carried out to examine the effects of both animal and plant-based low-carbohydrate diets on total mortality. Based on 6 prospective cohort studies including 5 million person-years of follow-up, compared to higher-carbohydrate diets predominated by refined sources of carbohydrate, high animal-based and plant-based low-carbohydrate diet scores were associated with a 13% increased and 17% decreased risk of total mortality, respectively [Figure 52] (331 332). Only the NHANES cohort examined the effect of a high-quality low-fat diet score. In this study, a high score was associated with a 27% decreased risk of total mortality (332). Importantly, however, this finding is compatible with the observations from a recent meta-analysis including 8.2 million person-years of follow-up indicating that each 15 gm/day increment of whole grain intake was associated with a 6% reduced risk of total mortality relative to other commonly consumed foods (44). Importantly, as whole grains were commonly defined as foods containing as little as 25% whole grain or bran content, this is likely a conservative estimate of benefit (333). It should also be recognized that the differences in intake of animal protein and fat between low and high dietary scores in these studies were only modest. However, as a dose-response relationship with changes to diet scores and total mortality was observed in several studies, this indicates that greater increases in an animal-based low-carbohydrate score would have been associated with an even greater increased risk (331 332).
While major health authorities have generally agreed on a benefit of substituting saturated fat with polyunsaturated fat, and to a lesser extent monounsaturated fat, the benefit of substitution with carbohydrate has been questioned by both health authorities and critics of plant-based diets alike, in part due to concerns that an increase in triglycerides, a reduction in HDL, and a possible increase in the mean diameter of LDL particles may offset the benefit of a lower concentration of LDL-C (4). However, recent evidence from over 100 clinical and genetic studies has established that it is the concentration of apo-B, representing the total number of atherogenic particles that is the primary lipid determinant of atherosclerotic cardiovascular disease and total mortality, with little or no benefit of modifying the size of lipoprotein particles, or raising HDL, independent of apo-B [Figures 37, 51, Table 1] (34 67 68 83 99). As established by a meta-regression analysis of over 100 controlled feeding experiments, substituting saturated fat with carbohydrate results in a meaningful reduction in apo-B [Table 12] (34). Moreover, a recent Mendelian randomization study involving over 1 million participants and 137,000 vascular events found that circulating arachidonic acid, a potential surrogate marker of saturated animal fat intake which associates with apo-B, LDL-C, triglycerides, apo-A-I in the same direction as that predicted for the substitution of carbohydrate with saturated fat, was causally associated with an increased risk of atherosclerotic cardiovascular disease proportional to the absolute increase in apo-B [Figure 37] (166). Thus, based on changes to blood lipids alone, substituting saturated fat with carbohydrate is predicted to reduce the risk of both cardiovascular disease and total mortality. Indeed, a recent Cochrane review of randomized controlled trials indicated that substituting saturated fat with either polyunsaturated fat or carbohydrate reduced the risk of combined cardiovascular events, proportional to both the absolute reduction in blood cholesterol and duration of exposure to lower intake [Figure 36] (164). A benefit of substituting saturated fat with carbohydrate is also indicated by the findings from prospective cohort studies examining cause-specific mortality, as well as a large body of classical evidence from international comparison studies and experiments in numerous species of nonhuman primates, including the chimpanzee [Figures 32-34, 53, Table 3-9] (170 171 172 334 335 336).
Evidence indicates that saturated fat intake also adversely affects multiple non-lipid risk factors. A recent Cochrane review of randomized controlled trials lasting at least two years found that reduced saturated fat intake resulted in a long-term reduction in both body weight and BMI (164). A review of 239 controlled dietary experiments found that substituting either monounsaturated or polyunsaturated fat with saturated fat adversely affects glucose-insulin homeostasis (337). Numerous controlled dietary experiments have found that substituting either unrefined sources of carbohydrate or unsaturated fat with saturated fat causes endothelial dysfunction (234 235 236 237 238 239 240 241 242 243). A recent review of clinical and epidemiological evidence found that saturated fat intake reduces bacterial abundance, diversity, and richness in the gut (338). In addition, a recent clinical trial also found that substituting major sources of saturated fat with walnuts reduces blood pressure (339).
Evidence indicates that saturated fat intake also increases the risk of non-cardiovascular mortality. A recent dose-response meta-analysis of prospective cohort studies including over 11 million person-years of follow-up found that in addition to increasing the risk of total and cardiovascular mortality, saturated fat intake was also associated with an increased risk of cancer mortality (169). Meta-analyses including up to 13 million person-years of follow-up have also found that saturated fat intake was associated with an increased risk of other major causes of death, including breast, ovarian, and lung cancer, Alzheimer's disease, dementia, and hip fracture (340 341 342 343 344 345). Several recent large studies have also found that substituting carbohydrate with saturated was associated with a substantially increased risk of respiratory disease mortality. Therefore, for this review, a meta-analysis was carried out to examine this effect. Based on data involving 647,000 participants with 10.7 million person-years of follow-up from 3 prospective cohort studies, substituting carbohydrate with a high intake of saturated fat was associated with a 66% increased risk of respiratory disease mortality [Figure 53] (19 346). Mendelian randomization studies have also found causal evidence that apo-B and LDL-C increase the risk of COVID-19, breast cancer, Alzheimer's disease, and bone fractures, indicating that many of these observed adverse effects are both likely causal and in part mediated by apo-B (280 281 322 347 348 349 350 351). An adverse effect on non-cardiovascular mortality is further supported by a recent Mendelian randomization study involving over 1 million participants which found that 42% of the causal effect of LDL-C on premature death was independent of coronary heart disease and ischemic stroke (98). As evidence strongly indicates that saturated fat intake has a fairly uniform effect across multiple major causes of death, this emphasizes the necessity of evaluating competing risk of death, and whether this may have obscured the association with certain disease endpoints, including cardiovascular disease.
A recent Cochrane review of randomized controlled trials found that reduced saturated fat intake significantly reduced the risk of cardiovascular disease, but while numerically lower, was not found to significantly reduce the risk of total mortality (164). However, the lack of a statistically significant benefit may in part reflect insufficient statistical power to detect a benefit as a result of a limited number of events, a limited reduction in intake, or a short duration of exposure to lower intake. Similar explanations have been given for the failure of clinical trials of smoking cessation to reduce the risk of total mortality until at least post-intervention (8 9). Indeed, the estimate for total mortality in the Cochrane review was similar to that observed in the Whitehall Smoking Cessation Study, the only apparent clinical trial on a healthy population that considered the effects of smoking cessation alone on mortality (8). Needless to state there has never been a definitive dietary or smoking trial carried out. Importantly, however, as was found for cardiovascular events, the between-study heterogeneity observed for mortality in the Cochrane review may also in part be explained by changes to blood lipids, which differed by up to 1.4 mmol/l between trials [Figure 36]. Therefore, a meta-regression analysis was carried out based on the data available from the Cochrane review to examine the effect of reducing saturated fat intake on the risk of total mortality proportional to the absolute reduction in total cholesterol. In a leave-out-one sensitivity analysis that excluded the Sydney Diet-Heart trial, the main contributor to between-study heterogeneity which accounted for less than 2% and 1% of deaths and total participants, respectively, the association with total mortality was statistically significant in both fixed and random-effects models. Based on 9 clinical trials involving over 55,000 participants and more than 3,400 deaths, a 1 mmol/l reduction in total cholesterol primarily through a reduction in saturated fat intake was associated with a 19% reduction in total mortality (RR=0.81 [0.65-1.00], OR=0.74 [0.55-0.99]) [Figure 54]. This is a similar magnitude of effect observed for the two clinical trials that observed a post-intervention benefit of smoking cessation on mortality in patients with a preexisting smoking-related disease (RR=0.83 [0.73-0.96], OR=0.81 [0.69-0.95] for the fixed-effect model) (9 352).
It is important to recognize that in the Sydney Diet-Heart trial saturated fat was evidently substituted in part with industrial trans-fat, which is observationally associated with an increased risk of mortality [Figure 4] (173). However, some authors have argued that the finding of lower total cholesterol levels observed in the saturated fat reduction group indicates a lack of an increase in trans-fat intake, playing on the common misconception that trans-fat intake necessarily increases total cholesterol levels (173). As established by a recent meta-analysis of controlled feeding experiments, substituting saturated fat with trans-fat actually reduces total cholesterol levels, in part due to reductions in HDL (37). Therefore, the substitution of saturated fat with trans-fat, as opposed to with purely linoleic acid, can actually help to explain the lower total cholesterol levels observed in the reduction group in this trial. In addition, the contrasting findings for circulating linoleic and arachidonic acid from Mendelian randomization studies over 2,000-fold the size in terms of both number of participants and cardiovascular events provides further confidence that the primary findings of this trial were likely either confounded by trans-fat or by chance (165 166 353). Taken together, the evidence indicates that the amount of saturated fat reduction, reflected by changes in serum cholesterol, and the quality of foods that displace sources of saturated fat can explain most of the heterogeneity between trials. The clinical evidence that saturated fat reduction reduces the risk of total mortality appears to be at least as consistent as that for smoking cessation. And as with smoking, it would likely be deemed unethical to carry out a definitive dietary trial and, hence, clinical evidence will likely always be limited.
Epidemiological evidence of an adverse effect of saturated fat on total mortality is indicated in both a recent dose-response meta-analysis of prospective cohort studies examining intake in primarily healthy populations, and in additional studies examining intake in diabetics (19 169 346 354 355 356 357 358 359 360 361 362 363 364 365 366 367). For this review, multiple additional studies not included in previous meta-analyses were identified. Therefore, an updated meta-analysis was carried out to examine the effect of saturated fat intake among participants who were either generally healthy, or who had been diagnosed with a cardiometabolic risk factor, but otherwise considered healthy at study baseline. For studies that measured intake per unit of change, estimates were calculated to represent a 5% increase in energy intake, otherwise, the estimates for high compared to low intake were considered, and where data permitted, converted to represent a 5% increase in energy. When a study examined the effect of substitution for specific macronutrients, the estimates for substitution for carbohydrate were considered. Based on data involving 1.4 million participants with 19.3 million person-years of follow-up from 24 prospective cohort studies, substituting approximately 5% of energy primarily from refined sources of carbohydrate with saturated fat was associated with a highly significant 9% increased risk of total mortality, independent of several potential intermediate risk factors including BMI [Figure 55]. The association with mortality was more pronounced when using the fixed-effects model as opposed to the random-effects model, reflecting a greater level of confidence of an increased risk reported in the largest studies (RR=1.13 [1.12-1.14]).
A subgroup analysis of prospective cohort studies indicated a more pronounced adverse effect of saturated fat for studies primarily carried out in Western nations than for Asian and developing nations (RR=1.12 [1.09-1.15]). The lack of a significant adverse effect observed in studies carried out in Asian nations contrasts that of clinical and genetic evidence indicating that lowering LDL, even down to low levels is causally associated with a reduced risk of total stroke, total cardiovascular events, and total mortality in Asian populations (69 174). As such, these discrepancies may reflect several major differences pertaining to dietary patterns between populations. Notably, this may partly reflect the time-lag effect of a shorter duration of exposure to higher intakes of saturated fat in many Asian and developing nations. It is well established that the time-lag for asymptomatic progression of atherosclerosis in response to moderately elevated apo-B before exceeding the burden threshold size that would result in a major cardiovascular event is up to several decades (32). Indeed, up until more recent decades which saw a major shift in dietary patterns towards a Western-type diet, the burden of atherosclerosis in many Asian and developing nations was comparatively low [Tables 8-9] (97 113). These discrepancies may also reflect residual confounding from socioeconomic status, which is highly correlated with the intake of animal foods in both developed and developing Asian nations (368). As low socioeconomic status is consistently associated with a greater increased risk of total mortality in many Asian and developing nations relative to Western nations, partly reflecting greater inequality gaps, residual confounding from socioeconomic factors would inevitably have a more pronounced effect on biasing the findings from studies carried out in these nations (369 370 371). As this particularly strong effect of socioeconomic status may more than compensate for the relatively small between-individual differences in intake of animal-based nutrients, residual confounding from socioeconomic factors may bias the findings towards a benefit for higher intake, indicating a necessity of caution when interpreting dietary findings from epidemiological studies carried out in these nations.
These discrepancies may also reflect measurement error. With the exception of the China Health and Nutrition Survey (CHNS) which found an association with an increased risk of mortality, all of the included studies carried out in Asian and developing nations only measured dietary intake at study baseline (367). Between-individual differences of exposure levels at study baseline are generally greater than the actual differences over time, frequently leading to an underestimation of the true association [Figure 2]. Indeed, in a more recent analysis from the CHNS, it was found based on eight dietary surveys carried out across a 20-year period that when measured as cumulative intake throughout adult life, but not at a single time point, a higher-fat lower-carbohydrate diet (%E) was associated with an increased risk of obesity, diabetes, and total mortality, with similar observations found for dietary cholesterol (372 373).
The 13% increased risk of total mortality observed for saturated fat in the fixed-effects meta-analysis is not unsimilar to the predicted effect of substituting 5% of energy from carbohydrate with saturated fat based on changes to apo-B for 5 years of cumulative exposure [Figure 51, Table 12]. As most studies only measured between-individual differences in intake at study baseline, this meta-analysis was not likely powered to detect a greater adverse effect for a longer duration of intake. Taken together with the evidence of overadjustment for several intermediate risk factors, including BMI, adverse changes to apo-B may explain almost all of the observed increase in risk of mortality. However, and importantly, as this meta-analysis examined the effect of substitution for predominantly refined sources of carbohydrate, the observed magnitude of effect cannot necessarily be extrapolated to other, potentially more appropriate sources of energy. Therefore, for this review, additional meta-analyses of prospective cohort studies were carried out to evaluate the effect of substitution with other major sources of energy.
No studies examining the effect of substituting saturated fat with unrefined sources of carbohydrate on the risk of total mortality were identified, although studies have reported a reduced risk of coronary heart disease when substituted specifically with carbohydrate from whole grains (334). Multiple studies examining the effect of substituting saturated fat with polyunsaturated fat on the risk of total mortality were identified. Therefore, for this review, a meta-analysis was carried out to examine this effect. In a meta-analysis of 7 prospective cohort studies including 11.4 million person-years of follow-up, substituting 5% of energy from polyunsaturated fat and omega-6 fatty acids with saturated fat was associated with a 23% and 22% increased risk of total mortality, respectively [Figure 56]. These findings are compatible with a broad range of evidence from clinical, genetic, epidemiological, and primate studies of a benefit of substituting saturated fat with linoleic acid, the predominant omega-6 fatty acid derived from grains, legumes, nuts, and seeds [Figures 4, 32-33, 36-38, 47, 51, Tables 1, 5, 12] (374).
Multiple studies indicate that monounsaturated fat (MUFA) has differential associations for animal and plant sources of food, likely in part reflecting the observation that animal sources of MUFA strongly correlate with saturated fat intake (346 354 355 356). Therefore, for this review, meta-analyses were carried out to examine the differential effects of plant and animal sources of MUFA intake on total mortality. In a meta-analysis of 6 prospective cohort studies including 10.1 million person-years of follow-up, substituting approximately 5% of energy from prominently refined sources of carbohydrate with animal MUFA was associated with a 12% increased risk of total mortality [Figure 56]. In addition, substituting 5% of energy from plant MUFA with animal MUFA, saturated fat, and a combination of animal MUFA and saturated fat was associated with a 30%, 19%, and 24% increased risk of total mortality, respectively [Figure 56]. Similarly, in a study including 7.3 million person-years of follow-up, substituting 1-tablespoon per day of butter with the equivalent of canola oil and olive oil was associated with an 11% and 16% reduced risk of respiratory disease mortality, and a 6% and 7% reduced risk of total mortality, respectively (364).
Both direct evidence from over 30 clinical trials and prospective cohort studies involving 1.4 million participants examining dietary intake, and indirect evidence from over 200 clinical and genetic studies involving 2 million participants examining apo-B-containing lipoproteins strongly indicates that substituting either high-quality sources of carbohydrate, polyunsaturated fat, or monounsaturated fat with saturated fat substantially increases the risk of total mortality [Figures 4, 51, 54-57]. Taken together, the overwhelming evidence from over one thousand clinical, genetic, epidemiological, and primate studies evaluating both risk factors and hard disease endpoints, for both cardiovascular disease and other major causes of death, and in both humans and nonhuman primates, has established beyond reasonable doubt that substituting high-quality plant foods with major sources of saturated fat substantially increases the risk of death from all-causes combined [Figures 1-56, Tables 1-14].
 |
| Figure 57. Effect of animal sources of monounsaturated fat and relative risk of all-cause mortality in a meta-analysis of 6 prospective cohort studies including 10.1 million person-years of follow-up |
There is a substantial body of high-quality evidence that the adverse effects of substituting high-quality plant foods with animal foods are in part independent of the effects of animal fat. Evidence from over 100 controlled dietary experiments has established that substituting plant protein with animal protein adversely affects blood lipids and glycemic control in humans [Figure 12] (40 375). The substitution of plant protein with animal protein has also been found to accelerate the development of atherosclerosis in laboratory animals, including nonhuman primates [Figures 35, 42]. In addition, evidence from clinical, genetic, and epidemiological studies strongly indicates that substituting high-quality sources of plant protein with animal protein increases the risk of cancer mortality [Figures 46-50].
The adverse effects of animal protein are also strongly supported by the findings from multiple large prospective cohort studies that substituting plant protein with animal protein increases the risk of total mortality (18 307 308 309 376 377). A recent analysis of the NHANES cohort found that substituting 2% of energy from plant protein with the equivalent energy from either unprocessed red meat or dairy protein was associated with a 35% increased risk of total mortality. Similarly, substituting 1% of energy from plant protein with the equivalent energy from seafood protein was associated with a 17% increased risk of total mortality (377). Moreover, substituting 5% of energy from plant protein with total animal protein was associated with a 106%, 94%, and 89% increased risk of total, heart disease, and cancer mortality, respectively, indicating that the adverse effects of animal protein intake are not likely only additive, but also multiplicative. However, this evidence has not yet been reviewed in the form of a meta-analysis. Therefore, for this review, meta-analyses were carried out to examine the effect of substituting plant protein with total and specific sources of animal protein on the risk of total mortality. Based on data involving over 750,000 participants with 13 million person-years of follow-up from 6 prospective cohort studies, substituting 3% of energy from plant protein, primarily from refined foods, with either total or individual sources of animal protein, including red meat, poultry, fish, dairy, and eggs was associated with between a 6% and 28% increased risk of total mortality [Figure 58].
The critics commonly argue that the observational evidence indicating an adverse effect of animal food intake on health outcomes can be explained by residual confounding from an unhealthy lifestyle and diet. This argument ignores a very substantial body of evidence from clinical, genetic, and primate studies that substituting high-quality plant foods with animal foods adversely affects health outcomes. Moreover, prospective cohort studies have also found that compared to sources of plant protein, animal foods associated with healthy lifestyles, including white meat, are also associated with an increased risk of total mortality [Figures 58]. For example, based on evidence from 4 prospective cohort studies including 5.1 million person-years of follow-up, substituting 3% of energy from plant protein with fish protein was associated with an 18% increased risk of total mortality [Figure 58]. Importantly, significant effects were observed in cohorts from both the U.S. and Japan, indicating an adverse effect in the context of a background of both low and high fish intake.
An adverse effect on mortality has also been observed for other animal foods commonly associated with a healthy diet and lifestyle. A recent analysis of the Nurses’ Health Study and Health Professionals Follow-up Study found that substituting one serving per day of whole grains and nuts with yogurt was associated with a 12% and 28% increased risk of total mortality, respectively [Figure 59] (378). While these findings do not necessarily negate a possible benefit of increasing intake of certain nutrients commonly derived from animal foods, such as long-chain omega-3 fatty acids, it does, however, strongly indicate the importance of deriving these nutrients from healthier plant-based alternatives. These findings also strongly indicate that the benefits frequently observed for animal foods commonly perceived to be health-promoting can largely be explained by the displacement of other suboptimal foods.
Consistent with the findings for animal-based macronutrients, animal-based micronutrients have also been associated with an increased risk of total mortality. In particular, multiple large cohort studies have found a dose-response relationship between dietary cholesterol intake and an increased risk of total mortality (303 311 357 373 379 380). For this review, a meta-analysis was carried out to examine the effect of dietary cholesterol intake among participants who were generally classified as healthy at study baseline. For studies that measured cholesterol intake per unit of change, estimates were calculated to represent a 300 mg/day increase in intake, otherwise, the estimates for high compared to low intake were considered, and where data permitted, converted to represent a 300 mg/day increase in intake. Where available, estimates based on maximum adjustment for intake of other nutrients were considered, with priority given to estimates adjusted for saturated fat. Based on data involving 1.3 million participants with 18.2 million person-years of follow-up from 22 prospective cohort studies, an approximately 300 mg/day increment in cholesterol intake was associated with a 16% increased risk of total mortality [Figure 60]. These findings were not materially altered after excluding studies that did not control for saturated fat intake [Figure 60]. These findings were also similar for both studies primarily carried out in Western and non-Western nations (RR=1.17 [1.13-1.20] and RR=1.21 [1.01-1.46], respectively). Moreover, the magnitude of increased risk was greater than that predicted by changes to apo-B, consistent with other lines of evidence indicating that the adverse of dietary cholesterol are both independent of and in addition to changes to blood lipids [Figures 11, 51, Tables 2-4].
In contrast to the findings for animal-based nutrients, dose-response meta-analyses of prospective cohort studies indicate that modest increments in intake of nutrients that often act as surrogate markers of high-quality plant food intake, including dietary fiber and antioxidant phytochemicals, were associated with between a 6% and 30% decreased risk of total mortality [Table 13] (44 175 176 384 385). These findings were generally most pronounced in studies examining circulating biomarkers of dietary intake which may help mitigate measurement error associated with dietary questionnaires commonly used in observational studies [Table 13]. Nevertheless, as with studies that examined intake using dietary questionnaires, most studies that examined intake using circulating biomarkers only measured exposure at study baseline, and thus, may still have underestimated the long-term benefits of minimally processed plant food intake.
It should also be recognized that the magnitude of reduced risk of mortality observed for a high intake of plant-based micronutrients is greater than that observed for linoleic acid in both studies examining intake using dietary questionnaires and circulating biomarkers [Table 13] (374). This is consistent with other lines of evidence that the benefits observed for most refined sources of unsaturated fat in the background of a Western-type diet are largely confined to reductions in apo-B, and that greater benefit, including improvements to multiple non-lipid risk factors which have additive or multiplicative effects on other risk factors, would likely be achieved when dietary fat is derived primarily from minimally refined foods rich in fiber and antioxidant phytochemicals, such as nuts, seeds, and soy [Figures 4, 32-33, 36, 38, Tables 2-3, 5, 12-13]. Furthermore, these findings indicate that a plant-based ketogenic diet that inadvertently strips much of these nutrients away to minimize the carbohydrate content of foods would also result in a higher residual risk of mortality compared to an unrefined plant-based diet richer in carbohydrate content.
 |
| Figure 61. Effect of choline intake and relative risk of all-cause mortality in a meta-analysis of 6 prospective cohort studies including 5.3 million person-years of follow-up |
 |
| Table 13. Meta-analyses of plant-based nutrients measured as dietary or circulating concentration and relative risk reduction of all-cause mortality |
Prospective cohort studies evaluating the long-term effects of substituting specific animal foods with high-quality plant foods also provide strong evidence that a diet rich in animal foods may increase the risk of premature death by more than two-fold [Table 14]. Most recently, the 16-year follow-up of the NIH-AARP study involving over 521,000 participants and more than 129,000 deaths found that substituting one whole egg per day with a serving of nuts and legumes was associated with a 13% and 10% reduced risk of total mortality, respectively. The substitution with one serving of nuts was also associated with a 30% reduced risk of respiratory disease mortality (303). These were greater benefits than observed for the substitution with any major source of animal protein. However, while this study was exceptionally large, diet was only measured at study baseline, and participants were recruited at an advanced age (mean age = 62.2), potentially leading to an underestimation of benefit through regression dilution bias and survivorship bias. By comparison, the findings from the Nurses' Health Study (NHS) and Health Professionals' Follow-Up Study (HPFS) which repeatedly measured diet every four years with validated food questionnaires throughout up to more than 30 years of follow-up, indicate that substituting one serving per day of high-quality plant foods with minimally processed animal foods, including unprocessed red meat and even yogurt may increase the risk of total mortality by up to more than 25% [Table 14].
While as with diet, the estimates for cigarette smoking and the risk of chronic disease and mortality may often be subject to underestimation as a result of imprecise measurements of long-term exposure, observational studies on smoking most often do not control for dietary factors other than alcohol intake, even when dietary data is available, and despite consistent evidence that smokers have poorer quality diets [Table 14] (386). Therefore, the estimates observed for diet which most often control for smoking and other major covariates may be more frequently subject to underestimation than that for cigarette smoking. Taken together, the overwhelming evidence from clinical, genetic, and epidemiological studies indicates that compared to a high-quality plant-based diet, a diet very rich in animal foods may increase the risk of premature death by a magnitude comparable to that observed for high-intensity cigarette smoking [Table 14] (16 70 311 312 313 314).
While the overwhelming evidence from clinical, genetic, and epidemiological studies strongly indicates a substantial benefit of a high-quality plant-based diet for reducing the risk of major causes of death, the critics often cite several prospective cohort studies that failed to demonstrate an association between vegetarian diets and a lower risk of total mortality to argue that plant-based diets confer no overall benefit. However, these criticisms often fail to recognize that vegetarians are not a homogenous group, and diets can vary greatly in quality. Additionality, the critics often fail to recognize several other important limitations typically inherited by such study designs, including reverse causality, and an absence of quantification of duration of adherence to a vegetarian diet.
In the study of smoking, it is considered important to not only examine participants current smoking status, but also their ever-smoking status. This can in part help researchers to examine the influence of reverse causality relating to the sick-quitter effect, where participants quit smoking in response to either a diagnosis of a disease, or unfavorable risk factors that would eventually result in adverse health outcomes. However, the quit-sicker effect has also long been found to influence diet. Even in the earliest prospective cohort studies examining diet dating back to the 1950s, participants were already found to have been limiting the intake of cholesterol and saturated fat in response to being diagnosed with high cholesterol, obscuring the association between diet and blood cholesterol levels and, in turn, the association between diet and the risk of cardiovascular disease (387). Despite these observations, this limitation has been entirely overlooked in many major relevant reviews on diet (171). Importantly, the quit-sicker effect has also been found to influence the adoption of plant-based diets. For example, in one cross-sectional study, it was found that 75% of the vegetarian participants with cancer adopted a vegetarian diet post-diagnosis, consistent with other research which found that cancer survivors are highly motivated to adopt a more plant-rich diet with the intention of improving poor health (388 389).
In the study of smoking, it is also considered important to examine the duration of smoking cessation, as it is well established that the magnitude of benefit is determined by the duration of cessation (313). However, it is well established that the effect that diet, and risk factors modified by diet have on health outcomes is also importantly determined by the duration of exposure [Figures 7, 21, 37, 51] (164). Importantly, several cohort studies have found that a longer adherence to a vegetarian diet was associated with a greater reduction in mortality. In a cohort of Seventh-day Adventists, very long-term compared to a shorter duration of adherence to a vegetarian diet was associated with a greater reduced risk of premature death [Figure 62] (390). In the pooled analysis of the EPIC-Oxford and Oxford Vegetarian cohorts, an association between a vegetarian diet and a lower risk of total mortality only became apparent after excluding participants who modified diet throughout follow-up (391). It is also important to recognize that the average length of adherence has been found to significantly differ between different types of vegetarian diets, with in general, shorter adherence the more restrictive the vegetarian diet. It was found in a recent study that among current vegetarians, pesco-vegetarians had been following the diet for 20% longer than lacto-ovo vegetarians, and more than 50% longer than vegans (392). Inevitably, this difference in duration of adherence would likely bias the findings from studies towards showing a greater benefit for less restrictive plant-based diets.
 |
| Figure 62: Cumulative survival, and life expectancy for very long and shorter-term vegetarians (17 years compared to <17 years) in the Adventist Studies. From Singh et al., 2003 |
Another important limitation of studies on vegetarian diets is that often only the effect of which animal foods are restricted is examined, and not the quality of plant foods displacing animal foods. It was found in a recent study that compared to omnivores, vegetarians consumed more ultra-processed foods, with greater intakes the more the restrictive the vegetarian diet (392). Therefore, the lack of significant benefit observed in some studies may in part reflect the substitution of animal foods with lower quality plant foods. To address these concerns, the healthful plant-based diet index (hPDI) was recently developed to examine the specific effect of diets richer in high-quality plant foods (393 394 395). In an analysis of the NHS and HPFS it was found both that the hPDI associated with a reduced risk of total mortality, and that the benefit increased over time with longer adherence (393). The hPDI has also been associated with a reduced risk of cardiovascular disease, prostate and breast cancer, type 2 diabetes, psychological disorders, hypertension, and erectile dysfunction [Figure 45] (248 249 294 393 394 396). Most recently, it was found in the COVID-19 Symptom Study involving over 592,000 participants and more than 31,000 cases that the hPDI associated with a 9% lower risk of developing COVID-19, and a 41% lower risk of developing severe COVID-19, independent of comorbidities, mask wearing, and community transmission rate (397). This is a magnitude of benefit similar to that observed for never compared to ever smoking (398). Importantly, the absolute benefit of a high-quality plant-based dietary pattern was particularly pronounced among participants living in areas with high deprivation who have been found to be at elevated risk (397). For this review, a meta-analysis was carried out to examine the association between the hPDI and total mortality. Based on 7 prospective cohort studies including 3.2 million person-years of follow-up, compared to a typical or habitual diet, a modest increase in the healthful plant-based diet index was associated with a 14% reduced risk of total mortality [Figure 63].
A recent systematic review involving over 508,000 participants and more than 42,000 deaths found that various plant-based dietary patterns were associated with a reduced risk of total mortality (399). While this analysis provides further evidence of the benefits of plant-based diets on reducing the risk of premature death, no recent meta-analysis has evaluated the association between vegetarian diets excluding all sources of flesh and total mortality in the context of long-term adherence. Therefore, for this review, a meta-analysis was carried out to examine the association between vegetarian diets and total mortality. Vegetarian status was either classified by self-classification status or reporting of flesh intake <1 time a month. Where possible, analysis was also restricted to subgroups of participants identified as not changing their diet status throughout follow-up, as well as subgroups of participants classified as healthy at study baseline. Based on 8 prospective cohort studies including approximately 3 million person-years of follow-up, compared to an omnivorous diet, adherence to a vegetarian diet of non-specific quality was associated with a modest, but statistically significant reduced risk of total mortality [Figure 63] (390 391 400 401 402 403).
It should be recognized that the studies which failed to find a benefit of a vegetarian diet had important limitations that potentially limited the ability to detect a possible benefit. In the Health Food Shoppers Study, a validity assessment of the survey used to classify the participants vegetarian status indicated that 34% of the self-reported vegetarian participants actually consumed meat (390). In the Heidelberg Study, the non-vegetarian group was predominantly semi-vegetarians, and differences in intake of meat between the two groups were therefore minimal. Nevertheless, this study found that very low meat intake for ≥20 years compared to <20 years was associated with a 29% decreased risk of total mortality, providing further evidence of a benefit of long-term restriction of meat intake (390). In the 45 and Up Study from Australia, one of the authors reported previously receiving funds from the meat industry. While this does not necessarily negate the findings of this study, it is peculiar that the authors chose to include participants with preexisting disease in their primary analysis, generally intended for inclusion in meta-analyses, raising concerns of a possible influence of the quit-sicker effect. Indeed, the authors found that at least 25% of the self-reported vegetarians were reported to have preexisting cardiovascular disease, cancer, or diabetes prior to the baseline dietary assessment (403). Additionally, despite the authors claim that the sample size was a strength of the study, only 0.63% of the population were self-reported vegetarians. Taken together with the relatively short duration of follow-up, this study had far fewer person-years of follow-up for vegetarian participants than any other included study [Figure 64].
A recent analysis evaluating the consumption of major foods and nutrients across 195 countries concluded that suboptimal diet is now responsible for more deaths globally than any other risk factor, including cigarette smoking (404). Diet has also recently been implicated as an important determinant of the risk and severity of respiratory diseases, including COVID-19, which has become a major contributor to the global burden of premature death [Figure 53] (19 303 346 364 383 397). Taken together with the overwhelming evidence from clinical, genetic, and epidemiological studies reviewed here, these findings reinforce calls for dietary improvements to be made a primary focus for reducing the global burden of premature death [Tables 13-14]. A substantial body of evidence indicates that within the context of a high-quality plant-based diet designed to sufficiently meet nutrient requirements, any increment in intake of animal foods above zero, including foods commonly recommended as part of a healthy diet, can increase the risk of premature death [Figures 7-15, 22-23, 30-31, 51, Tables 4, 12-14]. Comparatively, even small increments in animal food intake may increase the risk of premature death by a magnitude comparable to that observed for secondhand and low-intensity cigarette smoking, with higher intakes increasing the risk by a magnitude comparable to that observed for high-intensity smoking [Figures 49, 51, 54-60, Tables 13-15]. While low-carbohydrate high-fat diets rich in animal foods have become increasingly popular in large due to promises of weight loss, when compared specifically to a high-quality plant-based diet, the evidence not only indicates a lack of superior fat loss, but also that comparatively, adopting such a diet for weight loss is likely as dangerous as initiating smoking with the same intentions.
Protecting Life and Limb: The Athletic Advantage
It has been established beyond reasonable doubt that a high-quality plant-based diet is the most optimal diet for cardiovascular function [Figures 5, 7-45, 51, Tables 1-12]. As cardiovascular function is an important determinant of exercise capacity, plant-based diets may also benefit athletic performance. The Game Changers explores a number of the cardiovascular-related benefits of a plant-based diet on athletic performance, with an apparent emphasis on improved blood flow. Improved blood flow has been hypothesized to improve athletic performance via a number of mechanisms, including increased oxygen and nutrient delivery to the muscles (404). Indeed, it was recently demonstrated in a randomized, placebo-controlled trial that increased arterial blood flow improved performance recovery between bouts of high-intensity exercise (405). The documentary in particular emphasizes the large body of evidence of the benefits of plant-based foods on improving endothelial function, an important determinant of blood flow regulation. Compatible to the evidence reviewed, low carbohydrate diets rich in animal protein and saturated fat have specifically been found to impair endothelial function, particularly in comparison to a high-quality plant-based diet [Figures 43-44]. Moreover, the magnitude of adverse effect is comparable even to that observed for cigarette smoking (232 233).
There is a substantial body of high-quality evidence that the cardiovascular benefits of a plant-based diet extend to the limbs, likely translating to improved athletic performance. Flow-mediated dilatation (FMD), an index of endothelial function, is commonly measured in the limbs, and therefore an important predictor of arterial flow to these limbs. Clinical evidence has found both that cholesterol-raising diets rich in animal protein and saturated fat impair, and that cholesterol-lowering therapies improve FMD, indicating that apolipoprotein B (apo-B)-containing lipoproteins is an important determinant of endothelial function [Figure 44] (226 227 228 229). Experiments in nonhuman primates have also found that prolonged exposure to diets rich in cholesterol, saturated fat, and animal protein induces atherosclerotic plaque in most major arteries [Figures 33-35, 42]. Importantly, cessation of these diets has been found to result in the regression of atherosclerotic plaque formation in arteries that are major arterial supplies to both the arms and legs [Figures 33-34]. In one particular experiment, compared to a cholesterol-free diet, the feeding of even very small amounts of dietary cholesterol to monkeys resulted in the development of atherosclerosis in major arterial supplies to the limbs after only 18 months, independent of differences to LDL-C [Table 4, Figure 65] (130).
Evidence from clinical, genetic, and epidemiological studies also strongly indicates that cholesterol-lowering therapies reduce the risk of peripheral artery disease (PAD), a common circulatory disease in which narrowed arteries reduce blood flow to the limbs, most commonly the legs (406 407). A recent double-blind, placebo-based clinical trial found that lowering LDL-C from 94 mg/dl to 31 mg/dl not only resulted in a 27% reduction in risk of major cardiovascular events, but also reduced major adverse limb events by 42% in patients with PAD (408 409). Remarkably, there was no evidence of a threshold at which further reduction in LDL-C did not provide greater benefit, down to even below 10 mg/dl (0.26 mmol/l), and without major adverse effects [Figure 66]. Clinical trials have also found that cholesterol-lowering therapies improve walking distance more than other common drug types in patients with claudication, a condition involving exercise-induced cramping pain in the legs, commonly caused by PAD (410).
A recent randomized controlled trial coauthored by Caldwell Esselstyn found that a plant-based diet reversed vascular dysfunction and improved other various measures of PAD (254). In contrast, it has been observed that populations that subsist on minimally processed, low-carbohydrate diets, very rich in naturally derived animal foods have high rates of PAD [Table 11]. Moreover, a recent Mendelian randomization involving 412,000 participants and over 3,300 cases found that genetically predicted circulating arachidonic acid, a potential surrogate marker of saturated animal fat intake, was causally associated with an increased risk of PAD (411). Thus, the totality of evidence from clinical, genetic, epidemiological, and primate studies strongly indicate that the cardiovascular benefits of a cholesterol-lowering plant-based diet extend to the limbs and, in turn, likely improves athletic performance [Figures 33-34, 43-44, 65-66, Table 4].
 |
Figure 66. Effect of lowering LDL-C on the risk of major adverse limb events in the FOURIER clinical trial. From Bonaca et al., 2018 |
Animal protein has been classified as being of superior quality to plant protein based on common measures of protein quality. This most notably includes the Digestible Indispensable Amino Acid Score (DIAAS), favored by the Food and Agriculture Organization of the United Nations, which measures the short-term absorption of amino acids in the digestive tract relative to essential amino acids requirements. However, an important limitation of common measures of protein quality is that these do not account for the long-term adverse effects of animal protein on determinants of physical function, including an increased risk of cardiovascular disease.
Evidence from over 100 controlled dietary experiments has established that substituting plant protein with animal protein adversely affects blood lipids [Figure 12]. Substituting about 25 gm/day, or about 5% of total energy from plant protein with animal protein increases apolipoprotein B by 5 mg/dl, predicting a 17% increase in the lifetime risk of cardiovascular disease [Figure 51, Table 12]. Multiple lines of evidence also indicate that the adverse effects on cardiovascular disease are in part independent of blood lipids. Notably, controlled dietary experiments have found that substituting plant protein with animal protein adversely affects glycemic control in humans, and accelerates the development of atherosclerosis in nonhuman primates over and above that predicted by changes to blood lipids [Figure 35] (377). Clinical and epidemiological evidence also indicates that substituting plant protein with animal protein raises IGF-1, which was causally associated with an increased risk of coronary heart disease and type 2 diabetes in a recent Mendelian randomization study involving over 1 million participants (273 274 275 412).
The adverse effects of animal protein on cardiovascular function are also strongly supported by the findings from multiple large prospective cohort studies indicating that substituting plant protein with animal protein increases the risk of cardiovascular disease mortality (18 307 308 376). However, this evidence has not yet been reviewed in the form of a meta-analysis. Therefore, for this review, meta-analyses were carried out to examine the effect of substituting plant protein with total and specific sources of animal protein on the risk of cardiovascular mortality. In meta-analyses including 5 prospective cohort studies with 12.7 million person-years of follow-up, substituting 5% of energy from plant protein, primarily from refined foods, with either total or individual sources of animal protein, including red meat, poultry, fish, dairy, and eggs was associated with between a 17% and 52% increased risk of cardiovascular disease mortality [Figure 67]. This indicates that substituting between 6% and 15% of energy from plant protein with major sources of animal protein increases the risk of cardiovascular mortality by a magnitude comparable to that observed in the Nurses’ Health Study for 50-pack years of cigarette smoking (311).
Common measures of protein quality are at best, over-simplistic, and at worst, highly dangerous. While the DIAAS indicates that animal protein may be more efficiently absorbed in the digestive tract, recent evidence from clinical trials indicate that when consumed in adequate amounts, plant protein has similar short-term effects as animal protein on muscle strength and accrual (413). In addition, evidence from clinical, epidemiological, and primate studies strongly indicates that comparatively, animal protein substantially adversely affects long-term cardiovascular function. Thus, the evidence indicates that while animal protein has a similar effect to plant protein on lean body mass in the short term, the long-term consumption of animal protein may deteriorate the overall efficiency in which oxygen and essential nutrients are delivered to the muscles and, in turn, adversely affects body composition and impairs athletic performance (404 405).
Contrary to the findings from some studies examining plant-based diets of non-specific quality, a broad range of evidence indicates that a well-designed plant-based diet may reduce the risk of bone fractures and, in turn, bone-related injuries. Several recent meta-analyses have found that the intake of vitamin C and carotenoids, which are surrogate markers of high-quality plant food intake, is associated with a decreased risk of bone fractures, consistent with experimental research indicating that oxidative stress causes bone loss (418 419 420). In contrast, a recent meta-analysis of prospective cohort studies found that the intake of saturated fat and animal-derived monounsaturated fat was associated with a 79% increased risk of hip fracture and a 129% increased risk of overall bone fractures, respectively (345). In addition, a recent Mendelian randomization study involving over 426,000 participants and 53,000 fracture cases found that circulating arachidonic acid, a surrogate marker of saturated animal fat intake, was causally associated with both a reduction in bone mineral density and an increased risk of hip fracture (421). Moreover, both clinical and genetic studies have found that higher LDL-C is causally associated with reduced bone mineral density, providing further strong evidence that animal fat adversely affects bone health [Figure 69] (348 349 350 351). Taken together with the recent evidence from both clinical and genetic studies indicating that a high calcium intake, including from dairy products does not reduce the risk of bone fractures, but likely increases the risk of cardiovascular disease and premature death, these findings raise considerable safety concerns as to the emphasis placed on dairy and other animal food intake for bone health (422 423 424 425).
Interestingly, while cholesterol levels have generally not been observed to be especially elevated, a high rate of osteoporosis and other bone-related disorders have been commonly observed in both the post- and pre-European contact Inuit consuming a traditional marine-based diet, moderately rich in both calcium and vitamin D (181 187). In contrast, markers of osteoporosis have been observed to be much rarer in populations consuming higher quality plant-based diets, even among women accustomed to numerous pregnancies and long lactation periods (426). While there may be genuine concerns that a lower body weight and a lower intake of certain nutrients often associated with the restriction of animal foods may adversely affect bone health, these concerns can evidently be mitigated through a well-designed weight-maintaining plant-based diet. In contrast, there is strong evidence that sources of saturated fat increase the risk of bone fractures, and that this effect is in part mediated by changes to LDL [Figure 69] (345 422). Nutrients, including calcium are delivered via the bloodstream and hence require a healthy cardiovascular system for efficient delivery (404 405).
 |
Figure 68. Reductions in LDL-C and C-reactive protein levels in clinical trials comparing statins with ezetimibe/statin combination. From Catapano et al., 2017 |
Direct evidence of a benefit of lower cholesterol levels on athletic performance is indicated by an analysis from the National Runners' and Walkers' Health Study follow-up study involving over 66,000 runners with 480,000 person-years of follow-up, which found that high cholesterol was associated with decreased running distance (427). Importantly, however, a benefit of cholesterol-lowering was not observed for runners diagnosed with high cholesterol taking cholesterol-lowering pharmaceutical agents, possibly due to drug-related adverse side-effects, such as exercise-related muscle complaints observed with statin therapy.
Direct evidence of a benefit of a high-quality plant-based diet on athletic performance is indicated by a recent analysis of 102,000 women from the Nurses' Health Study, which found that a 4-year change in the healthful plant-based diet (hPDI) score was associated with significant improvements to all the individual component of the physical component score (PCS) and mental component score (MCS) [Figure 70] (428). This included improvements to measures often considered as determinants of athletic performance, including physical functioning, physical role limitations, bodily pain, and vitality. In contrast, compared to a steady diet, a 4-year change in the unhealthful plant-based diet score was associated with worse scores, indicating that discrepancies between studies examining the effects of plant-based diets on athletic performance may largely be explained by the quality and duration of adherence to a plant-based diet. In addition, a recent study involving over 35,000 participants found that compared to an animal-based dietary pattern, a healthy dietary pattern rich in vegetables was associated with improved handgrip strength, a marker of overall body muscle strength and physical function, independent of physical activity, metabolic syndrome, and other lifestyle characteristics (429).
Numerous epidemiological studies carried out over the last 115 years have fairly consistently indicated a benefit of plant-based diets for both stamina and endurance (430). Most recently, several cross-sectional studies examining the endurance of vegans suggested greater aerobic power relative to body weight compared to age-matched omnivores (431). This effect was particularly evident in a meta-analysis carried out for this review [Figure 71]. While benefit may be partly attributable to lower body weight, this magnitude of benefit is at least similar to that observed for never compared to heavy smoking (432). Similarly, in an earlier controlled feeding trial coauthored by Dean Ornish, it was found that a plant-based diet combined with stress management resulted in a 44% mean increase in duration of exercise after only 24 days in patients with coronary heart disease (105). However, few randomized controlled trials have evaluated the effects of plant-based dietary patterns on athletic performance in healthy adults, and have almost exclusively been short-term trials often involving lacto-ovo vegetarian diets of unspecified quality (433). Thus, the lack of difference in various outcomes between omnivorous and vegetarian diets observed in these trials does not necessarily negate the evidence of a long-term benefit of a high-quality plant-based diet.
The high levels of exercise capacity observed in a number of populations that traditionally subsisted on a plant-based diet may provide additional suggestive evidence of a benefit on athletic performance (434 435 436). Exceptional levels of physical fitness and endurance have been frequently observed among the Tarahumara (or Rarámuri) of northern Mexico, renowned for their ability of long-distance running [Table 15]. It has been observed that many of the Tarahumara runners can cover a distance of 160 km at a sustained pace of 10-13 km/hr, with races often continuing for a period of two days and nights, reaching up to more than 240 km (150 miles) (434 435). Lumholtz and Schwatka, two explorers who visited the Tarahumaras during the late 1800s described remarkable cases of long-distance mail deliveries made on foot over rough terrain at high altitudes. Lumholtz asserted (437):
A man has been known to carry a letter in five days from Guazapares to Chihuahua and back, a distance of nearly 600 miles [966 km] by the road. Even considering shortcuts, which he, no doubt, knew, it was quite a feat of endurance; for he must have lived, as the Indians always do while travelling, on pinole [corn powder] and water only.
Schwatka similarly noted (438):
…a Tarahumari, who made the round trip with his thirty or forty pounds of mail provisions in just six days, resting Sundays in Chihuahua to see the bullfight. This distance is over 500 miles [800 km], half of it being on a rough and hazardous a mountain trail as any in the known world.
In comparison, the current 6-day race world record in mostly optimal track conditions at low altitude is 1,036.8 km (644.2 miles), indicating that unless significantly exaggerated, these achievements may have been of world record class (439).
The high levels of exercise capacity observed among the traditional living highland populations of Papua New Guinea is also remarkable considering their more moderately active lifestyle based on cultivating crops and their exposure to risk factors often considered detrimental to athletic performance. These risk factors include deteriorated lung function, explained by the exceptionally high exposure to harmful smoke throughout their daily lives (209 434). The highland populations have also typically been observed to consume as little as 3% of energy from protein as a result of a monotonous diet with a very heavy reliance on low-protein staples [Table 8]. This is an intake lower than that reported for almost any population, with certain exceptions (209 214 440). While evidence indicates that the highlanders can often maintain nitrogen balance on these traditional diets, suggesting that for many, these diets meet the absolute minimum requirements for protein intake, food is not always readily available, helping to explain frequent reports of nutrient deficiencies (441).
Roy Shephard, a professor in Exercise Science compared the findings of aerobic power of a number of populations that typically spend at least a portion of life at high altitudes [Table 15] (434). Shepherd suggested that when accounting for the effect of decreased oxygen at high altitudes, the New Guinea highland populations, and even the elder former Tarahumara runners were found to have either similar or significantly superior aerobic power to that of active Inuit hunters (434). While comparisons of exercise capacity between different ethnic groups should be interpreted with caution, as differences may partly reflect non-dietary factors, including genetics, these findings are nevertheless compatible with the observations from recent within-population studies indicating higher levels of aerobic power in vegans compared to age-matched omnivores [Figure 71, Table 15].
Perhaps the most striking observation of traditionally plant-based populations is the delayed decline in physical performance with age relative to that of industrialized nations. It was observed that at an average age of 52, the former runners of the Tarahumara maintained high levels of physical performance that was significantly superior to that of the younger civilized Tarahumara, unacculturated to the Tarahumara traditional lifestyle and diet [Table 15]. Similarly, in addition to demonstrating levels of fitness often even superior to that observed in trained military divisions from industrialized nations, the Papua New Guinea highland populations have also demonstrated a remarkable lack of decline in fitness levels with age, particularly among men [Table 16] (209 434). Given the observed high levels of physical performance maintained into later life of populations subsisting on low protein, but otherwise high-quality plant-based diets, there may be merit for a reevaluation of the influence of nutritional aspects on long-term physical performance other than that of dietary protein that currently dominates sports nutrition. Recent epidemiological evidence has also found that surrogate markers of high-quality plant food intake, including dietary fiber, carotenoids, and vitamin C, are associated with the prevention of age-related reductions in skeletal muscle and muscle strength, as well as a reduced loss of handgrip strength (442 443 444 445 446 447). The common practice of stripping these nutrients from foods and eschewing foods otherwise rich in these nutrients in order to maximize the protein content of diet may not necessarily result in optimal long-term physical performance. Indeed, a recent study found that substituting carbohydrate of non-specific quality with animal protein was associated with higher frailty, independent of physical activity and other lifestyle characteristics (448). Amino acids are delivered via the bloodstream and hence require a healthy cardiovascular system for efficient delivery (404 405).
 |
| Table 15. Aerobic power measured by estimated maximal oxygen consumption (VO2 max) in select populations |
 |
| Table 16. Physical fitness predicted by the Harvard Pack Test scores in a New Guinea highland population. From Sinnett and Whyte, 1973 |
Consistent with the findings from population studies, there is an ever-increasing number of world-class athletes who have achieved major sporting feats at an advanced age while adhering to a plant-based diet. The Game Changers describes the achievements of former and current world record holders Carl Lewis and Dotsie Bausch, who not only continued achieving at the Olympics and other international sporting events, but actually achieved the best performances of their career after adopting a plant-based diet while being at an advanced age for their discipline. Other notable achievements include Mike Fremont breaking world records for both the fastest half and full marathons over the age of 90, Serena Williams breaking the record in women’s tennis for oldest ranked number one, and Novak Djokovic breaking the record in men’s tennis for oldest year-end ranked number one, and more recently, most year-end rank one finishes (1 449 450 451). These observations are not necessarily coincidental, but rather compatible with and complement the broad range of evidence supporting the notion that a high-quality plant-based diet can delay the decline in cardiovascular function and improve various measures of physical health and fitness [Figure 69-70].
Direct clinical evidence also indicates benefit of a plant-based diet for the prevention of physical decline over unprocessed animal-based diets. Notably, a recent controlled feeding trial found that in addition to reducing apo-B, blood pressure, and CRP levels, an ad libitum minimally processed low-fat plant-based diet also resulted in greater body fat loss and better maintenance of fat-free mass in healthy young adults compared to an ad libitum minimally processed animal-based ketogenic diet (50). Taken together with the findings from multiple other controlled dietary experiments that animal-based ketogenic diets often result in poorer outcomes in changes to fat-free mass, including lean-body mass during weight-loss in young adults, even when combined with resistance training, and despite most often being compared to diets predominated by refined sources of carbohydrate, these findings raise considerable safety concerns as to the effect of these diets on body composition of older adults who are at a much greater risk of muscle-wasting disorders (452 453).
The current paradigm of sports nutrition which focuses almost entirely on the immediate and short-term effects of animal protein and other animal-based nutrients on nutrient absorption and physical performance may present a missed opportunity to meaningfully extend the duration of peak athletic performance and competitive lifespan of both elite and amateur athletes alike. The totality of evidence from clinical, genetic, epidemiological, and primate studies strongly indicates that a high-quality plant-based diet can improve multiple important determinants of long-term athletic performance, including cardiovascular function, body composition, and the prevention of physical decline. It is not only the absorption of essential nutrients, but also the maintenance of the cardiovascular system responsible for the delivery of these nutrients to the bones and muscles that importantly determines the long-term effect of diet on physical performance. The totality of evidence calls for a reevaluation of common measures of protein quality to take into consideration the long-term adverse effects of animal protein on cardiovascular function and physical decline, especially given the strong evidence that the adverse effects extend to all-cause mortality [Figures 12, 35, 42, 58, 67]. Taken together with other findings described here, there is convincing evidence that a high-quality plant-based diet is optimal for both longevity and the prevention of physical decline. Not only have these diets been found to be cost-effective, the evidence indicates that a high-quality plant-based diet may literally save an arm and a leg (454).
The Elephant in the Room
Ever since the birth of modern medicine with Hippocrates almost 2,500 years ago evidence has been mounting that diet is an important determinant of health. At the dawn of the 20th century, following continuous reductions in deaths from communicable diseases, it was becoming increasingly evident that the majority of the population in the industrialized world was now succumbing to largely preventable noncommunicable diseases, including cardiovascular disease, cancer, and type II diabetes (113 208 266). The global geographic distribution in incidence of these diseases has since been commonly observed to range from between 10- to more than 50-fold, and highly correlated with differences in dietary intake [Figures 72-73, Table 7] (455 456 457 458 459). It has been over 110 years since Williams demonstrated a correlation between diet and the global geographic distribution of cancer, over 90 years since Anichkov demonstrated the progression of atherosclerosis can be stopped in laboratory animals by diet, over 85 years since Rosenthal demonstrated a correlation between diet and the global geographic distribution of cardiovascular disease, over 60 years since Keys demonstrated in metabolic ward experiments that blood cholesterol can be modified by diet, over 50 years since Armstrong demonstrated atherosclerosis can be reversed in primates by diet, over 40 years since Peto demonstrated in a meta-analysis of randomized controlled trials that blood cholesterol has a dose-dependent effect on coronary heart disease, and over 30 years since Ornish demonstrated that atherosclerosis can be reversed in humans through dietary and lifestyle changes alone.
Already half a century ago, a number of programs aiming at improving dietary quality were being implemented at the population level in response to the mounting evidence. These programs were often followed by large declines in cardiovascular mortality and increases in life expectancy [Figures 25, 27]. The North Karelia Project which started in 1972 as a national pilot program for Finland was one of the most successful of such programs [Figures 25, 27] (460). By the 1960s, the Finnish population which subsisted on diets very rich in fatty meat and dairy had the highest rate of cardiovascular mortality in the world and a low life expectancy relative to other developed European nations. Mortality rates were especially high in North Karelia in Eastern Finland, prompting the launch of the project which included a key aim of improving the quality of dietary fat, particularly through a reduction in dairy fat, and later, an increase in fruit and vegetable intake. Life expectancy subsequently increased by 11 and 9 years in men and women, respectively, with half of this increase explained by a reduction in cardiovascular mortality, and despite a doubling of smoking prevalence among women over the same period (461 462). Ironically, and despite risk factor models predicting that continued favorable changes to multiple risk factors would not be wiped out by recent adverse dietary changes for at least another several years, some of these same regions, including North Karelia, that experienced among the greatest declines of cardiovascular mortality in the world following dietary improvements, are for the first time in decades showing indications of an increase in incidence, particularly among the younger age groups who have been misled to believe that diets rich in “meat, butter, and cheese” are health-promoting (335 463 464 465). As the world has witnessed with the outbreak of the Coronavirus disease 2019 (COVID-19) pandemic, reducing adherence to science-based recommendations as a result of compliance fatigue or any other reason will not solve global pandemics of either communicable or noncommunicable diseases.
There is clearly a significant discordance between the published evidence and the general public’s understanding of diet and health. While there is room for legitimate debate on certain effects of diet, the overwhelming evidence from thousands of studies has established beyond reasonable doubt that a high-quality plant-based diet, that is, a diet based primarily on minimally processed plant foods rich in fiber and antioxidant phytochemicals is optimal for both longevity and the prevention of physical decline [Figures 1-73, Tables 1-16]. As was once the case for cigarette smoking, this discordance may not necessarily reflect a lack of general interest in health, but rather, the success of numerous misinformation campaigns. Perhaps most relevant to this phenomenon is the tobacco industry playbook, which describes the tactics used by the tobacco industry beginning in the 1950s of spreading fear, uncertainty, and doubt in order to undermine scientific evidence and sway public opinion on the harms of cigarette smoke (466 467). This playbook is still evolving even to this day, if anything, at an accelerated rate with the advent of the information age. A renewed focus on defense mechanisms, including psychological projection, victim playing, and feigned outrage is evidently central to the modern-day version of this playbook, often employed by tobacco industry-linked researchers when confronted with the critical flaws in their arguments (468). Such tactics are often intended to promote bothsidesism to give the false impression that the available evidence more equally supports opposing viewpoints than otherwise indicated in attempt to dissuade the general public from making lifestyle changes to improve health. Given the evidence of the strong influence of this playbook on multiple industries, it merits examining whether the critics of plant-based diets, namely advocates of animal-based diets, the livestock industry, and to a lesser extent, major health authorities have employed similar tactics with the intention of amplifying the confusion in the general public surrounding animal foods and health (5 466 469).
 |
| Figure 72. Relationship between saturated fat intake at baseline and 50-year coronary heart disease death rates in men from the 16 cohorts of the Seven Countries Study. From Kromhout et al., 2018 |
 |
| Figure 73. Relationship between per capita daily meat consumption and colon cancer incidence in women in 23 countries. From Armstrong and Doll, 1975 |
The first step in solving a problem is to acknowledge that it exists. In this case, it is that the general public is exceptionally vulnerable to misinformation on diet and health (470). This vulnerability is not necessarily entirely the result of a lack of knowledge on the subject, but also importantly, the result of a number of cognitive biases and the means by which these are frequently exploited (471). That so many may be unable to even fathom, despite consistent evidence across clinical, genetic, epidemiologic, and primate studies, that diet may increase the risk of chronic diseases and mortality by a magnitude comparable to that of cigarette smoking demonstrates the robustness of these effects [Figures 1-74, Tables 1-16].
Confirmation bias is the cognitive bias that describes the tendency to actively interpret or search for information in a way that conforms to one’s preconceptions. It is clearly evident, perhaps more than many may be willing to admit, that information portraying animal foods in a positive manner is actively sought. People are now exposed to upwards of more than 5,000 advertisements per day with messages that attempt to influence our consumption behavior (472). Stated otherwise, people are on average being advised what to consume every waking minute of the day. Yet, few of these messages appear to cause so many the degree of psychological distress as the notion of restricting the consumption of animal foods (473 474 475 476). While this response is evidently the result of a number of factors, research indicates this may be fundamentally cultural. It has been observed in Western populations that those who abstain from animal products are evaluated more negatively than several common prejudice target groups, stemming in part from heightened perception of threat from engagement in antinormative behavior (475). It has also frequently been observed that there are cultural-dependent links between meat, particularly red meat consumption, and framings of masculinity, luxury, and social status, and that these framings impact the willingness to reduce meat consumption (473 474 477 478). These perceptions are not inherent in young children, but rather appear to be acquired as they become acculturated to Western ideology (479). However, even in adulthood, attenuation of these perceptions often attenuates preference for consumption (476). As a result of these perceptions, many may be more inclined to restrict the intake of other highly palatable foods and alcohol and make other non-dietary lifestyle changes than they are to restrict the intake of animal foods to improve health. Inevitably, for many, this will mean that certain diets, such as low carbohydrate diets that typically allow for an unrestricted intake of animal foods will be more appealing than other restrictive diets. Where there is demand there will be supply, and there is little doubt that the prospects of fulfilling this demand have not influenced many who promote an animal-based diet. Certainly, it hardly seems a coincidence that even bestselling authors frequently rebrand the animal-based diet they advocate soon after it becomes apparent that it is more lucrative to do so. The most notable recent trend is undoubtedly with the advocacy of Keto. The decrying of every major study that finds evidence of benefit of replacing animal foods with plant foods these advocates otherwise claim to promote does little to help the argument that these diets are not simply tailored to fulfill this demand. As is made clear from the preponderance of evidence on diet and health, the justifications to promote these diets are simply an afterthought [Figures 1-74, Tables 1-16].
Cognitive dissonance is the cognitive bias that describes the tendency of actively seeking consistency with a preconceived notion to minimize psychological distress resulting from a conflict with new information. Donning a thinking cap can often be more difficult than one made of tin foil. As with any human being, even scientists are not immune to cognitive dissonance, and there is a long history of medical practitioners rejecting highly efficacious therapies, particularly when acknowledging the benefits would require accepting that almost the entire population is unhealthy (64 480 481). This phenomenon has been argued to have contributed to the confusion between normal and optimal levels of blood cholesterol, resulting in many decades of lost opportunity before the scientific community near-universally embraced the evidence that optimal levels are far below that of the average person in the industrialized world [Figures 7, 24, 27, 30-31, 51, 66] (64).
Perhaps not entirely coincidental, a greater acceptance of risk factors traditionally modified by diet, such as blood cholesterol and blood pressure, evidently followed the wide availability of the respective pharmaceutical agents (64). For many, the discomfort of accepting the benefits of a pill may be easier to swallow than for the dietary changes required to achieve a similar magnitude of effect. Unlike many other lifestyle exposures, such as cigarette smoking, food is necessary to sustain life, and even scientists will have a particular dietary preference. Inevitably, cognitive dissonance may have resulted in a greater or lesser degree of skepticism applied to peer-reviewed publications on diet relative to other exposures depending on how the findings align with prevailing dietary preferences. The extent of this problem can in part be illustrated by the discrepancies in consideration of the survivorship bias. In the study of most harmful exposures, including dietary-modifiable risk factors, the common paradoxical phenomenon in which an exposure associates with an increased risk of mortality or a late-onset disease in mid-age, but then plateaus and eventually reverses with age will commonly be ascribed to the survivorship bias, in which the healthiest exposed participants are automatically selected for inclusion based on surviving competing risk of prior death (65 66 324 482). In contrast, when this phenomenon is observed for the intake of animal-based foods or nutrients, evidence of the influence of this bias is often entirely neglected in favor of arguing the hypothesis of benefit relating to differential dietary requirements of older adults (483).
The illusory truth effect is the cognitive bias that describes the tendency of believing misinformation in response to repetitive exposure. This effect has been found to be so powerful that it can even overwhelm rationality (484). The robustness of this effect has likely been amplified in recent years, both through the filter bubble effect, in which digital information is strategically repeated based on the users' preferences, and through the echo chamber effect, in which fragmented communities, including those that commonly exist on social media, share and repeat a particular narrative in a way that is insulated from rebuttal (485 486). As a result, people can often become largely isolated from the prevailing scientific consensus on certain issues. While well documented as being a target for exploitation by the tobacco industry, these phenomena have not only been targeted, but perhaps in many cases successfully exploited by the advocates of animal-based diets (335 487). The lack of prior knowledge of the existence of much of the relevant evidence documented within the literature cited here by many who have actively sought out information may be a testimony to this.
The present bias is the cognitive bias that describes the tendency of overvaluing short-term rewards while putting lesser worth on long-term consequences. It is not likely fully appreciated that there is a prolonged period, often of several decades, of asymptomatic progression of chronic diseases caused by harmful environment and lifestyle exposures before many chronic diseases are commonly diagnosed (31 32). Approximately half of all global deaths are caused by chronic diseases that most commonly progress asymptomatically, including cardiovascular disease and cancer [Figure 74] (27 31 32). Despite this, patients diagnosed with asymptomatic conditions often misjudge their state of health, leading to unnecessary dismissal of professional advice (488). Clearly, people do not know what works for their own bodies as well as many may think. Short-term benefits, perceived or actual in response to dietary change may also provide a false sense that a suboptimal diet is otherwise healthy, resulting in unwarranted doubt cast on evidence of the long-term adverse effects. Given that many popular diets restrict widely consumed highly-palatable foods, it should only be expected that for many this would result in caloric restriction-induced weight loss and, in turn, a number of weight-loss related benefits regardless of dietary composition (489 490). Similarly, many may also experience measurable short-term benefits in response to the placebo effect due to repetitive exposure to a particular narrative of diet. A notable example of this are the findings from placebo-based studies that a significant portion of patients perceived to have gluten sensitivity have been found to report discomfort in response to gluten-free foods perceived to contain gluten, and in contrast, a lack of discomfort when consuming gluten-containing foods not necessarily perceived to contain gluten (491 492 493). As has been recently highlighted by the dangers of presymptomatic and asymptomatic transmission of SARS-CoV-2, it is important to recognize that many diseases can progress asymptomatically, and that there are pitfalls of over-relying on symptoms when making educated decisions on diet and lifestyle (494).
 |
| Figure 74. Effect of primary and primordial prevention and the time-lag for the progression of atherosclerosis and risk of acute cardiovascular events. From Ference et al., 2018 |
The Dunning-Kruger effect is the cognitive bias that describes the tendency whereby people with limited knowledge or competence in a specific area overestimate themselves (495). Many things appear simpler than they actually are, and because one skill set is not necessarily transferable to another, even the highly skilled can be susceptible to this bias. Unfortunately, what people do not know can hurt them. Humans are by nature categorical thinkers, creating ad hoc categories to cope with complex subjects (496). It is, however, these categories that have contributed to much of the confusion and controversy surrounding diet and health. The study of diet is more complicated than often recognized in that it can generally only be elucidated in relative, not absolute terms. That is, foods can generally not be meaningfully categorized as having a specific effect on health, be it beneficial, neutral, or detrimental, without defining an appropriate comparison food.
In the lawsuit United States v. Philip Morris, the U.S. Department of Justice condemned a large industry-linked study by Enstrom and Kabat suggesting a null effect of secondhand smoking on smoking-related diseases as an example of “scientific fraud” (497). Of a number of flaws of this study, exposure misclassification is by far considered to be the most serious (14 25). Exposure misclassification was indicated to have resulted in part due to the lack of repeated measurements of exposure during late follow-up (14). Between-individual differences of exposure levels measured during early follow-up are generally greater than the actual differences over time, frequently leading to an underestimation of the true association (13). Most serious, however, exposure misclassification was indicated to have resulted from exposure being classified based on spousal smoking status of nonsmokers in an area and time in which exposure was near-universal. It is well documented that spousal smoking is a poor predictor of total secondhand smoke exposure in populations where exposure is near-universal, as virtually all the participants are exposed at some point in their daily lives (498). Essentially these researchers compared two groups subjected to a harmful exposure with only small differences in level of exposure, and as a result, observed little difference in the rate of disease between the two groups.
While their attempts to exploit defense mechanisms did little to negate the arguments that this study is an example of scientific fraud, these researchers argument that epidemiological studies of other exposures, including diet are subject to far less scrutiny despite inheriting some of these same problems may not be entirely without merit (468). In the study of diet, intake is most often only measured at study baseline, yet evidence of bias introduced by dietary changes throughout follow-up is almost entirely neglected in many major reviews (171). Similarly, as most commonly studied populations consume largely homogeneous diets, within-individual intake is often greater than between-individual intake, leading to further misclassification of between-individual intake (499 500). Arguably, however, the most serious problem plaguing the study of diet may also be largely comparable to that for the study by Enstrom and Kabat- the inadequately disclosed practice of comparing one suboptimal exposure to another. That is changes in intake of suboptimal foods, macronutrients, and dietary patterns are most frequently compared to habitual intake, being predominantly suboptimal sources of energy, without this being recognized in the conclusions (3 501 502). Whether different sources of energy have differential effects on overall health is no longer a subject for debate. Evidence from over 1,000 controlled dietary experiments has established beyond reasonable doubt that the effect that any source of energy has on health is importantly determined by what sources of energy are being substituted, and that substitution with different sources have differential effects on health [Figures 5, 8-15, 18, 20, 36, 44, 46, 54]. Recently, Walter Willett in response to a problematic review on dairy intake, emphasized concerns as to how this excessively common practice has likely misled the general public to falsely conclude a lack of benefit of restricting the intake of many suboptimal foods (3).
To conclude that dairy foods are ‘‘neutral’’ based on relative risks close to 1.0 could be misleading, as many would interpret this to mean that increasing consumption of dairy foods would have no effects on cardiovascular disease or mortality. Lost is that the health effects of increasing or decreasing consumption of dairy foods could depend importantly on the specific foods that are substituted for dairy foods.
The practice of neglecting the effect of dietary substitution has led to an exceptionally dangerous situation entering into the genetic age, pathing the way for the publication of research claiming causal beneficial effects of suboptimal foods based on genetic associations without recognizing that the observed effects reflect the displacement of other suboptimal foods. The extent of this problem can be illustrated by the problematic conclusions of publications examining lactase persistence genotypes as a genetic proxy of cow's milk intake. It is well documented that milk displaces predominantly suboptimal sources of energy, including soft drink intake (3 503). Most recently, it was found in the EPIC-CVD study comprising of cohorts from 8 European countries that each increase in the milk intake increasing allele was associated with a 7.3 g/day reduction in meat intake, representing an even greater change in energy than the 13.7 g/day increase observed for fluid milk intake (504). Nevertheless, these findings were not addressed in the authors conclusions which argued based in part on the associations for the same allele, a lack of causal evidence of an effect of milk intake on cardiovascular disease (504).
While neglect of the effect of dietary substitution in the literature examining genetically predicted dairy intake may be ubiquitous, when interpreted in the appropriate context these studies may still meaningfully contribute to the understanding of diet. In the PREDIMED trial, it was observed that the lactase persistence genotype was associated with a 44% and 74% increased risk of total mortality among all participants and women, respectively, in the intervention group advised to consume a higher-quality Mediterranean diet, but with little effect in the control group that received contemporary low-fat dietary advice that places minimal emphasis on replacing animal foods with high-quality plant foods (505). While not explored by the authors, these findings indicate that the more pronounced evidence of harm observed within the intervention group may have been a result of milk displacing higher quality foods consumed as part of a Mediterranean dietary pattern, complimenting the findings from prospective cohort studies that when substituted for high-quality plant foods, but not most other commonly consumed foods, dairy intake substantially increases the risk of total mortality [Figures 49, 59]. Nevertheless, in a meta-analysis carried out for this review involving 120,000 participants and over 17,000 deaths, compared to predominantly other suboptimal foods, genetically predicted cow’s milk intake was causally associated with a statistically significant increased risk of total mortality [Figure 75] (505 506 507 508). Importantly, these findings were largely independent of the genetic variant examined, and therefore unlikely to have been confounded by pleiotropic effects of individual variants [Figure 75]. Moreover, as all studies were carried out in the European Union (EU), this indicates that these findings were independent of the administration of bovine growth hormone due to a ban in place on use since 1990. These findings provide among the strongest causal evidence to date of a benefit of restricting the intake of dairy, primarily in favor of an increased intake of high-quality plant-based foods.
Mendelian randomization studies have also provided causal evidence to complement the findings from prospective cohort studies that compared to the intake of predominantly suboptimal foods, milk intake increases the risk of Parkinson’s disease, and in nations where intake is high, prostate cancer [Figure 76] (275 509 510 511 512 513). Genetically predicted milk intake has also been causally associated with an increased risk of obesity in both adults and adolescence, and in both developed and developing nations, confirming the findings of weight gain observed for dairy intake in a meta-analysis of ad libitum intervention trials lasting at least one year (514 515 516 517 518). Moreover, these studies also complement the findings from prospective cohort studies that even when compared to predominantly suboptimal foods, milk intake has no overall benefit on bone fracture risk (423 519 520).
 |
| Figure 75. Effect of cow's milk intake genetically predicted by lactase persistence genotypes and risk of all-cause mortality in a meta-analysis of Mendelian randomization studies including 120,000 participants and 17,000 deaths. Genotypes include lactase non-persistence (CC), lactase persistence heterozygous (CT), and lactase persistence homozygous (TT) |
 |
| Figure 76. Effect of cow’s milk intake and risk of Parkinson’s disease in a meta-analysis of 8 prospective cohort studies including 5.9 million person-years of follow-up and 2 Mendelian randomization studies including 420,000 participants and 20,000 cases |
While meaningful progress has been made, the practice of neglecting the effect of dietary substitution is still apparent even in recent publications by major health authorities that have refrained from setting a recommended upper level of intake for certain suboptimal foods. Most recently the American Heart Association (AHA) released updated dietary guidelines which emphasized the benefits of predominantly plant-based diets low in ultra-processed foods based on extensive evidence from substitution analyses (521). The guidelines also importantly took into consideration non-cardiovascular-related diseases, environmental impact including greenhouse gas emissions, and water and land usage, as well as disparities by socioeconomic status, race, and ethnicity (521).
The guidelines' emphasis on the importance of dietary substitution extends on an earlier advisory from the AHA of the benefits of reducing the intake of saturated fat for the prevention of cardiovascular disease, which specifically cautioned against the often flawed interpretations of studies reporting a null or near null association due to displacement with predominantly low-quality sources of energy (4). Nevertheless, concerns remain around the guidelines of a meaningful residual risk of chronic disease and mortality compared to a more focused high-quality plant-based diet. Notably, the emphasis on consuming low-fat and fat-free dairy intake was based primarily on substitution analyses with high-fat dairy, and does not appear to have adequately accounted for the evidence of further benefit for the substitution with high-quality plant foods [Figures 3, 12, 46, 49, 58-59, 67]. Also of concern, while emphasis was placed on consuming primarily plant-based sources of protein and restricting the intake of red meat, eggs were not specified as a food that should necessarily be restricted. This builds on the problematic conclusions of an earlier AHA advisory which examined the effects of dietary cholesterol on cardiovascular risk (522). In this advisory, the panel cited the findings of a near null association between egg intake and risk compared to non-specific sources of energy as a primary justification for not specifying a recommended upper level of intake for eggs (522). In doing so, the panel effectively argued a largely comparable effect of eggs with other low-quality foods that the AHA recommendations restricting as evidence of a lack of harm. The panel even specifically stated in their conclusions that vegetarians “may include more dairy and eggs in their diets” in the context of moderation. Consideration of direct and indirect evidence of the effect of substitution for high-quality plant foods recommended by the AHA would likely have proved considerably more difficult to justify these conclusions [Figures 11-12, 33-34, 37, 50-51, 57-58, 60-61, 67, 75-76] (3 303 523). Moreover, as indicated by an earlier meta-analysis of 76 controlled feeding experiments, dietary cholesterol has a greater effect on raising cholesterol levels with lower baseline intakes, suggesting the harms of increasing intake may be most pronounced for vegetarians and others consuming a habitually low cholesterol diet [Figure 77] (523).
The advisory panel also argued that the findings from their primary analysis of controlled feeding experiments examining the effects on blood lipids, including LDL, provided the most convincing evidence to date of an at most, modest harm of dietary cholesterol on cardiovascular risk. What the panel found was that dietary cholesterol raised LDL-C, although not necessarily significantly, in all 19 examined comparisons with low intake (522). Indeed, when using standardized meta-analysis methodology that would be commonly applied to a Cochrane review, the evidence that dietary cholesterol raises LDL-C would likely be considered of high certainly [Figure 78]. Despite this, the panel chose to cast doubt on the evidence of an adverse effect on LDL-C based on the significant statistical heterogeneity observed in the dose-response meta-regression model selected for analysis [Figure 79]. However, it is well established in controlled feeding experiments that different baseline intakes of dietary cholesterol and the inclusion of hypo- and hyper-responders will result in significant heterogeneity between comparisons [Figure 77] (524). Thus, evidence of statistical heterogeneity alone should not rule out a meaningful effect on blood lipids. Excluding only two comparisons predominated by hypo- and hyper-responders that contributed significantly to statistical heterogeneity would have shown a statistically significant dose-response effect of dietary cholesterol on LDL-C [Figure 79] (525 526).
It should also be recognized that the meta-regression model used by the AHA advisory panel differs importantly from that used in most other major publications examining the dose-response effect of diet on blood lipids, in that it allows for a zero change in intake to predict a non-zero change in blood lipids [Figures 8-11, 79] (37 39 527). The appropriateness of this model in the study of diet has been a cause of controversy, questioned even in major publications from the World Health Organization (39):
Regression lines were forced through the origin because a zero change in diet should produce a zero change in blood lipids.
Importantly, if the panel had only modified the analysis to force the regression line through the origin (where zero change in intake predicts zero change in effect), it would have shown a highly statistically significant dose-response effect of dietary cholesterol on LDL-C [Figure 79]. This model predicts that each 100 mg/day increase in dietary cholesterol increases LDL-C by 1.86 mg/dl, which is virtually identical to that observed in another recent analysis funded by the egg industry that the AHA advisory panel questioned the validity of [Figure 11] (39 522).
The question is clearly not whether increasing cholesterol intake from low levels increases LDL-C, but whether this increase translates into an increase in apolipoprotein B (apo-B), indicated by clinical, genetic, and epidemiological evidence to be the primary lipid determinant of atherosclerotic cardiovascular disease, by a magnitude that would meaningfully influence the risk of disease [Table 1]. This was one of the multiple important questions that went unanswered by the AHA advisory panel. While data for apo-B was available for only a subset of comparisons examined, the number of comparisons is identical to that considered by the panel for subgroup analyses for other measurements of blood lipids (522 526 528 529 530). Based on the same meta-regression model used as the advisory panel, but with the regression line forced through the origin, dietary cholesterol is associated with a statistically significant increase in apo-B [Figure 11]. These comparisons indicate that a 300 mg/day increment in dietary cholesterol, representing the upper limit previously recommended by the AHA, increases apo-B by about 3.5 mg/dl, predicting a 12% increase in the lifetime risk of cardiovascular disease [Figures 11, 51]. Doubling this to 600 mg/day, representing the 90th percentile of intake in the U.S., predicts a 25% increase in risk, a magnitude comparable to that observed for secondhand smoke exposure (16 531).
 |
| Figure 77. Effect of dietary cholesterol on total blood cholesterol at different baseline intakes in a meta-analysis of 76 controlled feeding experiments. From Hopkins, 1992 |
 |
| Figure 78. Effect of dietary cholesterol on LDL-C (mg/dl) in a meta-analysis based on the 19 comparisons identified by the American Heart Association (AHA) for the 2020 science advisory on dietary cholesterol and cardiovascular risk |
 |
| Figure 79. (A) Dietary cholesterol and changes to LDL-cholesterol (mg/dl) in a meta-regression analysis replicated from the model used by the American Heart Association (AHA) for the 2020 science advisory on dietary cholesterol and cardiovascular risk (P=0.335). (B) Same as (A) but excluding two comparisons that contributed significantly to heterogeneity (p=0.0383). (C) Same as (A), but with the regression line forced through the origin (P=<0.0001) |
Of the herd of elephants in the room in the study of diet, one is clearly of mammoth proportions. The conclusions of many studies of diet are presented in a misleading way, in that it is often difficult for researchers, let alone the general public to ascertain that the described effects of specific foods, macronutrients, and dietary patterns are compared to predominantly suboptimal sources of energy, and that these effects may differ, in some cases by an order of magnitude when compared to healthier sources of energy. In the information age where the general public has increasingly greater access to the abstracts from scientific journals, the continued practice of neglecting the effect of dietary substitution will only continue to contribute to the confusion surrounding diet and, in turn, do little to help solve the global pandemic of noncommunicable diseases. The normalization of this practice must be unanimously rejected in a similar manner as has been done for the use of inappropriate comparisons in the study of secondhand cigarette smoking. Greater emphasis must be placed on comparing foods to healthy alternatives to help determine optimal diets for health. It is not only appropriate, but essential to compare apples to oranges, because the health of the general public is not negotiable. While leading researchers have carefully pointed out this and other serious flaws in major publications on diet, the lack of universal condemnation has meant that the work of Enstrom and Kabat will be continued to be published in leading peer-reviewed journals, but under different high profile researchers names and for a different harmful exposure (3 4 22 171 501).
As is the case for the tobacco industry, doubt is the product of the critics of plant-based diets (466). The fact that there are always two sides to an argument simply means that there are two sides, not that both arguments are necessarily legitimate. By establishing controversy through the exaggeration of uncertainty, the critics attempt to give the general public the false impression that there is insufficient evidence to conclude a benefit of substituting animal foods with high-quality plant foods. Whether these arguments have any sense of legitimacy may be of little concern when the target audience has insufficient knowledge on the subject. This may especially be the case when the arguments conform to preconceptions of the target audience.
It is important to recognize denialism in the debate of scientific research, often defined as the employment of arguments to give the appearance of legitimate debate where none exists (6). While there is room for legitimate debate on the effect that diet has on a number of health outcomes, certain aspects have already been proven beyond reasonable doubt. This notably includes the unequivocal evidence that substituting high-quality plant foods with animal foods, particularly those rich in saturated fat increases the concentration of apo-B-containing lipoproteins. High-quality evidence indicates that when isocalorically substituted for equal parts of energy from carbohydrate, monounsaturated fat, and polyunsaturated fat, a 10% increase in energy from saturated fat together with a 600 mg/day increment in cholesterol intake increases apo-B by about 80.4 mg/dl, predicting a 100% and 187% increased risk of total mortality and cardiovascular disease, respectively, for 5 years of cumulative exposure [Figure 51, Table 12]. Ironically, despite the frequent calls by the advocates of animal-based diets to shift the focus away from LDL-C in favor of more advanced measures of lipoproteins that better predict risk, the concentration of apolipoprotein B, which represents the total number of circulating atherogenic particles and evidently the primary lipid determinant of atherosclerotic cardiovascular disease, actually predicts a greater increased risk of both cardiovascular disease and total mortality for increasing intakes of saturated fat (%E) than that for LDL-C [Figure 51, Tables 1, 12] (34 67 68 532 533 534). As it has been established that any mechanism of lowering apo-B reduces the risk of cardiovascular disease proportional to both the absolute reduction in apo-B and cumulative duration of exposure to lower apo-B, so long as there are no significant competing deleterious off-target effects, the advocates of animal-based diets are forced to either cite a very substantial body of high-quality evidence that the non-lipid benefits of increasing the intake of animal foods outweigh the adverse effects of higher apo-B, in addition to all other established non-lipid benefits of high-quality plant foods, or resort to denialism [Figures 7, 21-24]. Many have chosen the latter, perhaps owing to a lack of evidence of such benefit. A relevant example of this can be illustrated by the misinformation promoted by Chris Kresser, a proponent of the Paleo diet, and vocal critic of plant-based diets, who in a grossly inaccurate article attempted to refute the findings from over 500 highly controlled feeding experiments, in addition to numerous other lines of evidence that has unequivocally established that saturated fat (%E) raises blood cholesterol levels (35 36 535):

It should be recognized that the studies Kresser refers to as being “long-term” are actually observational cross-sectional studies evaluating the association between diet and cholesterol levels at a single point of time, and should not be confused with the actual forward-looking findings from the cohort studies from which these were derived (536). Nevertheless, as opposed to the claim of only one study, numerous other within-population cross-sectional studies have found a positive association between saturated fat intake and blood cholesterol levels (536 499). Moreover, it had already been mathematically demonstrated more than 40 years ago that these cross-sectional studies have virtually zero statistical power to detect a true relationship between diet and cholesterol levels due to various biases that these study designs typically introduce, and therefore very rarely cited in the scientific literature as evidence to refute the findings from controlled feeding experiments [Figure 80] (500).
Most importantly, however, despite claiming that the studies which found that saturated fat increased blood cholesterol were "almost always short-term, lasting only a few weeks", more than 30 controlled dietary experiments referenced in the 2003 meta-analyses cited by Kresser to back up this statement actually lasted between 5 weeks and up to 8 years, with more supporting studies published since [Figures 81-82] (4 35 537 538 539). Moreover, a Cochrane review involving over 7,000 participants from 13 randomized controlled trials each lasting at least 2 years found that reducing the intake of saturated fat reduced blood cholesterol over the long-term [Figure 83] (164). Kresser also ignored the evidence that reductions in saturated fat intake (%E) have been followed by both long-term reductions in blood cholesterol and cardiovascular mortality at a population level in many nations throughout the globe prior to the widespread use of cholesterol-lowering pharmaceutical agents [Figures 25-27] (85 540). Similarly, despite referring to animal studies in the same article, Kresser failed to recognize the large body of evidence from long-term experimental atherosclerosis studies demonstrating that animal-based nutrients, including saturated fat both raise blood cholesterol and accelerate the development of atherosclerosis in over 20 species of non-human primates, including the chimpanzee [Figures 32-35, Tables 3-5, 10-11]. This is a glaring omission coming from someone who claims to promote an evolution-based diet.
 |
Figure 80. Mathematical model showing that the findings from within-population cross-sectional studies typically have virtually zero statistical power to detect a relationship between diet and cholesterol levels. (A) The open circles represent the relationship that exists in five typical individuals between diet score and predicted serum cholesterol. Vertical arrows represent random variations in serum cholesterol and horizontal arrows random variations in diet scores. (B) The open circles demonstrate how the perfect correlation in A is completely obscured by the introduction of typical sources of intraindividual variability. From Jacobs al., 1979
|
 |
Figure 81. Serum cholesterol in the Veterans Administration Study over 8 years. Total blood cholesterol was reduced by 13%, and total cardiovascular events were reduced by 21% in the experimental group for which sources of saturated fat were substituted with omega-6 rich corn, soybean, safflower, and cottonseed oils. From Dayton et al., 1969
|
 |
| Figure 82. Serum cholesterol in the Oslo Diet-Heart Study over 5 years. Total blood cholesterol was reduced by 14% (41 mg/dl), and total cardiovascular events were reduced by 29% in the experimental group for which sources of saturated fat were substituted with polyunsaturated fat-rich vegetable oil. From Leren, 1970 |
 |
| Figure 83. Effect of reducing saturated fat on total blood cholesterol in a Cochrane review involving 7,113 participants from 13 randomized controlled trials, each lasting at least 2 years. From Hooper et al., 2020 |
Although when examined in the entirety, the evidence of benefit of a plant-based diet is arguably unequivocal, there is no single definitive study proving the efficacy of any specific diet on chorionic disease and mortality. While there may be near-universal agreement on the benefits of modifying a number of environmental and lifestyle exposures, including smoking habits, that have not been subjected to, or proven in rigorous randomized controlled trials, there is still much unwarranted controversy surrounding the benefits of plant-based diets due to an overemphasis on small evidence gaps (4). There have long been concerns that the controversies generated by unrealistic demands for definitive evidence by the protagonists of radical science-based medicine have unnecessarily subjected the general public to harm. These concerns have recently received renewed and widespread attention amid the largely unwarranted controversies surrounding the efficacy of the use of face masks and vaccines for the mitigation of the COVID-19 pandemic (541 542 543). Indeed, a recent Mendelian randomization study involving over 135,900 participants and more than 5,400 cases found that circulating arachidonic acid, a potential surrogate marker of saturated animal fat intake, was casually associated with a far greater increased risk of blood clot than that observed for any widely distributed COVID-19 vaccine (411). Clearly, dietary modification appears to be a more effective strategy to reduce the risk of the serious, but exceptionally rare COVID-19 vaccine side effects than vaccine avoidance. As has been highlighted in a humorous manner, many of the arguments that focus on small evidence gaps to oppose the adoption of certain interventions could likely as easily be applied to cast doubt on even the efficacy of parachute use in the prevention of death and major trauma for gravitational challenge (544 545). These arguments have notably included:
- Correlation does not equal causation. A number of interventions believed to be beneficial failed to show efficacy when subjected to randomized controlled trials, and therefore observational evidence indicating benefit of parachute use should be interpreted with caution
- Mortality from free fall from extreme heights is not 100%
- Efficacy of parachute use is not 100% and is associated with adverse effects due to failure of intervention
- Benefits of parachute intervention observed in nonhuman studies cannot necessarily be extrapolated to humans
- Confounding caused by a “healthy cohort” effect may exaggerate the benefits of parachute use, as people who jump from extreme heights without assistance of a parachute are more likely to have a psychiatric comorbidity or unhealthy lifestyles
- Industry-sponsored studies are more likely to favor their commercial product, questioning the reliability of any evidence sponsored by the “evil” billion-dollar parachute industry “whose profits depend on belief in the efficacy of their product”
The example of the parachute both highlights the limitations of hierarchies of evidence in evidence-based medicine and emphasizes the argument that even in the presence of multiple evidence gaps, recommendations can still be made for certain interventions, so long as the evidence meets a certain threshold for safety and efficacy. However, by dismissing a very substantial body of ever-increasing evidence supporting the benefits of a high-quality plant-based diet while simultaneously citing comparatively weak and misleading evidence to justify increasing the intake of animal foods, the advocates of animal-based diets have demonstrated that they are by no means protagonists of radical science-based medicine. They merely masquerade as such when it serves to justify their unrealistic demands for ever-higher quality of evidence for any findings not supportive of their narrative. The goalposts never rest. They are well aware that various challenges around feasibility, compliance, and ethics would make the type of definitive dietary trial they demand almost if not impossible to carry out. Ultimately, the advocates of animal-based diets have taken a page from the tobacco industry playbook to argue the necessity of idly waiting for a definitive dietary trial they know will likely never be carried out before their narrative can be refuted.
It was recently demonstrated in the first randomized controlled trial carried out on the subject, no benefit of parachute use for the prevention of death and major trauma when jumping from an aircraft. This was humorously achieved by having the participants jump from a stationary aircraft on the ground [Figure 84] (546). Although this critical flaw was made clear for even the casual reader, this has not been the case for many studies, which is in part what led the authors to emphasize that “accurate interpretation requires more than a cursory reading of the abstract". Clearly, absence of proof in a randomized controlled trial is not necessarily proof of absence. Unfortunately, clinical trials are not necessarily always designed to demonstrate efficacy, but often to hide adverse effects (547). While studies cannot necessarily be negated based on sources of funding alone, it is important to recognize flaws and design limitations that may bias the results in favor of a particular outcome. This may be particularly important for dietary experiments where the conclusions have been found to usually always be in favor of the funding source. For example, in one analysis of 78 dietary experiments on various beverages, including milk, it was found that 0% of industry-funded studies had an unfavorable conclusion as opposed to 37% of studies with no industry funding (548). As with most exposures, the effect of diet is importantly determined by three factors. The quantity of change in exposure, the duration of exposure, and the comparison exposure. Studies designed to hide evidence of harm of a particular food or diet in part by the intentional exploitation of one or more of these factors are about as informative as a trial of parachute intervention in which participants jump from a stationary aircraft on the ground, and only serves to show the funding industry’s lack of confidence in the health properties of their own product.
 |
| Figure 84. The PArticipation in RAndomized trials Compromised by widely Held beliefs aboUt lack of Treatment Equipoise (PARACHUTE) trial that “debunked” the widely held belief that free fall from an aircraft increases the risk of death or major traumatic injury compared to parachute intervention. From Yeh al., 2017 |
Finally, it is important to recognize the multifactorial nature of diet and noncommunicable diseases. Advocates of animal-based diets often argue that processed foods are the primary dietary determinant of chronic diseases in an apparent effort to downplay the adverse effects of animal foods. This plays on both the appeal to nature fallacy and the fallacy of the single cause (335). While evidence supports a benefit of replacing ultra-processed foods with minimally refined plant-based foods, these foods alone cannot explain the entire burden of diet on chronic disease and premature death [Table 13]. Many dietary-related diseases have been observed in humans predating the availability of ultra-processed foods by thousands of years, and in animals predating humans by 100 million years [Figures 39, 41, Tables 6-7] (203 204). In epidemiological studies, a high burden of dietary-related diseases has been observed among traditionally living populations subsisting on minimally processed, low-carbohydrate diets, rich in naturally derived animal foods [Figures 39-41 Tables 6-7]. In primate studies, diets composed virtually entirely of processed foods, with intakes of up to 77% and 40% of energy from refined sugar and omega-6-based vegetable oils, respectively, have been used to successfully reverse atherosclerosis induced by minimally processed animal foods, including egg yolks [Figures 33-34, Table 5]. In genetic studies, cow's milk intake has been causally associated with an increased risk of total mortality compared to predominantly other low-quality foods, independent of growth hormone use [Figure 75]. Perhaps most importantly, in clinical trials, substituting sources of saturated fat primarily with refined sources of polyunsaturated fat and carbohydrate has been found to reduce the risk of total cardiovascular events and total mortality proportional to the absolute reduction in blood cholesterol [Figures 36, 54]. Nevertheless, these diets have still been observed to result in a significant residual risk of chorionic diseases compared to intervention studies using a whole-foods plant-based diet [Figures 28-29]. This highlights the important fact that simply because a particular diet may reduce the lifetime risk of the single greatest global cause of death by up to 50% in the background of a Western-type diet, it does not necessarily establish it as an optimal diet. The answer for the other 50% is a whole-foods diet, not based on animal foods, but plants.
A common propaganda technique is the use of loaded language to invoke an emotional, rather than a rational response (549). In attempting to exploit a lack of rational response from their targeted audience, this allows the propagator to dismiss unfavorable evidence without the necessity of citing sufficient or even relevant counterevidence. It, therefore, merits attention as to why the advocates of popular animal-based diets have often chosen to use loaded language when dismissing a very substantial body of unfavorable evidence. With the explosion in popularity of plant-based diets in recent years, everything from documentary films to prominent studies from Harvard emphasizing the benefits of a plant-based diet are now frequently dismissed as Chris Kresser chose to describe The Game Changers, “vegan propaganda” (550). Likewise, for long have leading researchers who contributed to the traditional dietary recommendations to restrict the intake of animal-based nutrients been dismissed, as prominent Paleo diet advocate Robb Wolf chose to describe, “dumb”, for apparently grossly misinterpreting the evidence and failing to understand basic concepts in their own field of expertise (551).
To give the benefit of the doubt that many of these arguments have been made in good faith would often require conceiving that these advocates have somehow unintentionally overlooked thousands of relevant studies [Figures 1-82, Table 1-16]. Both the arguments that animal-based nutrients do not raise blood cholesterol, and that blood cholesterol does not increase the risk of cardiovascular disease can each be refuted by over one thousand pieces of evidence [Figures 5, 7-15, 21-27, 30-37, 51, 54, 74, 77-83, Tables 1-5, 12] (535 551). Similar is the case for the argument that recommendations to restrict the intake of animal foods are even remotely based on the findings from the early atherosclerosis experiments in rabbits, which Robb Wolf, for example, argued had seemingly been extrapolated to humans despite significant genetic differences and their herbivorous nature (551). Not only does this ignore that such extrapolations were virtually never made, even by the authors who published these very experiments, but such arguments also appear to ignore the existence of thousands of experiments that largely replicated these findings across over 100 different species and breeds of animals, including omnivores, carnivores, and more than 20 species of primates [Figures 32-35, Tables 3-5, 10-11] (64). This is a glaring omission coming from someone else who claims to promote an evolution-based diet.
The totality of evidence strongly, and in some cases, overwhelmingly supports many of the proposed, often controversial benefits of a plant-based diet argued by several mainstream documentary films. These include the wide range of benefits described in The Game Changes, such as a reduced risk of cardiovascular disease, cancer, type II diabetes, obesity, and perhaps most controversially, improvements to athletic performance and erectile function [Figures 1-83, Table 1-16]. These include the controversial arguments described in Forks Over Knives that a plant-based diet can help reverse heart disease, and that animal protein is a major cause of cancer [Figures 3-15, 28-37, 46-51, Tables 1-9, 12]. And when considered in the appropriate context, these also include the highly controversial argument in What The Health that the intake of a single egg can increase the risk of premature death by a magnitude comparable to that of smoking 5 cigarettes. As indicated by a broad and substantial body of evidence, when compared specifically to high-quality plant foods, modest increments in intake of most minimally processed animal foods, including, eggs, red meat, chicken, fish, and dairy can increase the risk of premature death by a magnitude comparable to that of a modest increment in cigarettes smoked [Figures 1-83, Tables 1-14]. To simply dismiss many of the arguments of the benefits of a plant-based diet as “vegan propaganda” despite the vast body of supporting evidence would imply either the existence of a very substantial body of high-quality contrary evidence, or that this term is simply being used as a propaganda technique to compensate for a lack of contrary evidence. However, while there may be room for legitimate debate on whether there are benefits of including small amounts of animal foods in an otherwise high-quality plant-based diet, the evidence simply leaves no room for interpretation that a diet other than one that is predominantly plant-based is optimal for the health of the general population [Figures 1-83, Tables 1-16]. Every accusation is a confession. The advocates of animal-based diets have voluntarily taken a page from the tobacco industry playbook and are projecting the shortcomings of their own arguments in feigned outrage.
As a definitive dietary trial does not, and may never exist, all lines of evidence must be carefully evaluated in the context of both the strengths and limitations, without exaggerating either. Simply identifying limitations of a particular study does not necessarily negate the fact that the findings may still meaningfully contribute towards the understanding of diet and health. This argument was a focus of a recent review by several leading nutritional researchers, including Walter Willett, commissioned to address the multiple serious false claims being circulated relating to the classical findings of Ancel Keys from the Seven Countries Study (335 336). The major dietary findings from the Seven Countries Study, including the observation that the intake of saturated fat, dietary cholesterol, meat, butter, milk, and combined animal foods were major determinants of heart disease and total mortality has been seen as a major target for many advocates of animal-based diets, whose arguments have shown to be consistent only in terms of lack of accuracy [Figure 72] (335 336 455 456 552 553 554). Certainly, the conclusions drawn by Willett and colleagues may very well describe the means by which these advocates attempt to dismiss most unfavorable lines of evidence.
Like all studies, SCS [Seven Countries Study] should be analyzed and utilized in context, with strengths and weaknesses acknowledged. By deliberately or carelessly misrepresenting historical events, distorting scientific findings, and misstating researcher intent, modern critics of the SCS routinely impede, rather than advance, understanding in nutritional epidemiology.
The paradox of tolerance describes the idea that without limit, tolerance will lead to the ability to be tolerant destroyed by the intolerant, and to maintain tolerance, it is therefore necessary to be intolerant of intolerance (555). For the same reasons, caution must be exercised to ensure the ability to debate the effect of diet is not impeded as a result of unlimited tolerance to those with no intention of participating in legitimate debate. It is abundantly clear. The advocates of diets rich in animal foods, including the so-called Keto, Paleo, and Carnivore diets have ignored, suppressed, and misrepresented thousands of relevant studies from more than a century of research, which when considered in the totality, indicate that compared to very low or possibly no intake, small increases in animal food intake can meaningfully increase the risk of chronic disease and premature death. These adverse effects are, in many cases, multiplicative with both the change and cumulative duration of intake, and without evidence of a threshold. The claims that the intake of animal foods per se do not adversely affect disease risk factors or cause chronic diseases implies that these advocates have either succumbed to the Dunning-Kruger effect, and do not understand the fundamental principle of dietary substitution, or are deliberately attempting to mislead the uninformed. The overwhelming preponderance of evidence from over 100 million person-years of follow-up from clinical, genetic, and epidemiological studies, over 1,000 controlled dietary experiments, and thousands of experiments in over 100 species and breeds of animals, including more than 20 species of primates provides compelling support for the notion that compared to a minimally processed plant-based diet rich in fiber and antioxidant phytochemicals, long-term adherence to diets rich in animal foods can significantly accelerate the development of cardiovascular disease and cancer, cause sexual dysfunction, impair athletic performance, and increase the risk of premature death by a magnitude comparable to that of high-intensity cigarette smoking [Figures 1-83, Tables 1-16]. Keto in its popular form is not just the new Paleo or Atkins. Keto is the new tobacco.
While there are obviously key differences between dietary and smoking habits, there are striking similarities for both the certainty of evidence, and the evidence of the magnitude of effect on multiple important health outcomes, including the risk of premature death. As with cigarette smoking, the evidence of the magnitude of effect of diet on health is far too great to simply stand idle while awaiting evidence from a definitive dietary trial that will likely never be carried out. At the very least, to the extent it may give a sense of urgency to the pandemic of noncommunicable diseases, it does not seem entirely unjustified to equate animal foods with smoking. It is past time that a well-designed plant-based diet is made a primary focus for the prevention and treatment of chronic and degenerative diseases, and to promote healthy longevity. While there are clearly other important healthy lifestyles practices that should complement a healthy diet, these alone cannot fully compensate for a suboptimal diet rich in animal foods. The truth can hurt, and advocates of animal-based diets will continue to use every trick in the tobacco industry playbook to exploit this fact and profit from selling comforting lies. The constant and unrelenting repetition of misinformation they have spread has done nothing to change the overwhelming body of evidence but has nevertheless served its intended purpose- to confuse the general public. This in turn will only help ensure that diet remains the number one global cause of premature death. For this reason, among others described here, they must be called out in the same manner that Government institutes have called out tobacco industry-linked researchers Enstrom and Kabat. While the names of the diets may be various, the advocates of animal-based diets can be divided into two types of people; those who are misinformed, and those whose intent is to defraud the misinformed.








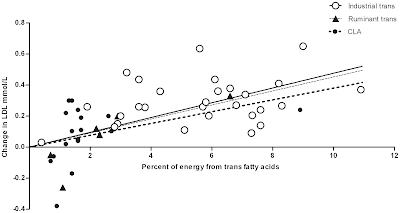

























































Social Plugin